Figure 1.
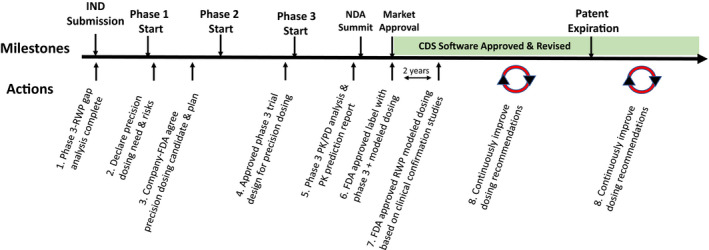
Precision drug dosing recommended actions over the investigational new drug (IND) and new drug application (NDA) Life Cycle. There are eight proposed actions over the research and development drug cycle designed to produce more precise dosing recommendations at market approval or soon thereafter and continuously improve dosing based on real‐world data (RWD). CDS, clinical decision support; FDA, US Food and Drug Administration; PD, pharmacodynamic; PK, pharmacokinetic; RWP, real‐world patient.
