Figure 7.
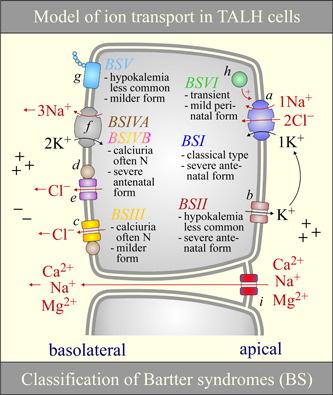
Model of ion transport and causes of NKCC2 dysfunction in the TALH. Ion transport: K+ recycling through K+ channels (b) in the apical TALH and through K+ channels (not shown) and the Na+/K+ ATPase pump (f) in the basolateral TALH cause both the luminal and serosal fluid to be positively charged (Greger, 1985; Greger & Schlatter, 1981; Hamilton & Devor, 2012; Hebert & Andreoli, 1984; Hebert, Culpepper, & Andreoli, 1981; Hurst, Duplain, & Lapointe, 1992). However, outwardly directed Cl− conductive pathways (c–e) in the basolateral TALH decreases the effect of K+ recycling on the serosal side such that active NaCl reabsorption in this nephron segment causes the luminal‐to‐serosal transepithelial potential to increase along with the paracellular reabsorption of certain cations (Gu et al., 2009; Guinamard, Chraibi, & Teulon, 1995; Winters, Zimniak, Mikhailova, Reeves, & Andreoli, 2000; Yoshitomi, Koseki, Taniguchi, & Imai, 1987). NKCC2 dysfunction: Different types of Bartter syndromes (BS) are listed along with an overview of their clinical manifestations (see Cunha and Heilberg (2018) as to why these manifestations vary among the types). The color of the headings “BS” match those of the proteins affected. BSIVB appears in various shades given that it is associated with pathogenic mutations in both CLCNKA and CLCNKB. In this figure, the salt‐losing nephropathy that has been linked to melanoma antigen D2 has term BSVI arbitrarily. The proteins shown are (a) NKCC2 (SLC12A1), (b) ROMK2 or ROMK3 (KCNJ1), (c) CLCNKB, (d) Barttin, (e) CLCNKA, (f) the Na+/K+ ATPase, (g) Ca2+‐sensing receptor, (h) melanoma antigen D2, and (i) claudins (isoforms 14, 16, or 19). N, normal; NKCC2, Na+–K+–Cl− cotransporter 2; TALH, thick ascending loop of Henle
