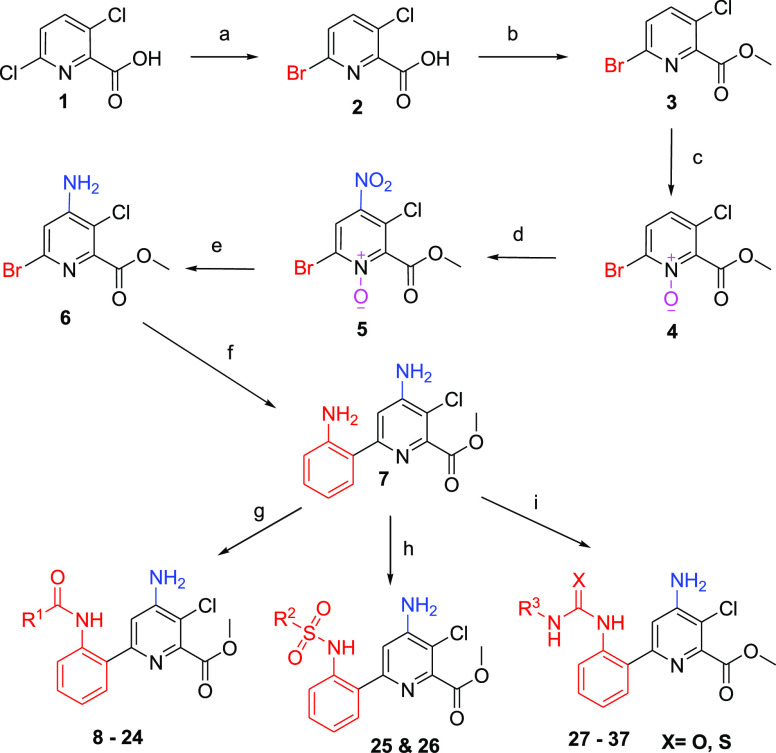
Scheme 1. Synthesis of Methyl 4-Amino-6-(2-aminophenyl)-3-chloropicolinate Amide, Urea, and Thiourea Derivatives.
Reagents and conditions: (a) HBr in acetic acid, AcOH, 110 °C, 24 h; (b) methanol, H2SO4, reflux, 6 h; (c) trifluroacetic acid, TFAA, H2O2, 80 °C, 3 h; (d) HNO3: H2SO4, 70 °C, 4 h; (e) Raney Ni, MeOH, 45 °C, H2, 8 h; (f) Pd(II)Cl2(dppf), K2CO3, 2-aminophenyl boronic acid, EtOH, toluene, 90 °C; (g) R1COCl, DCM, 6 h, RT; (h) R2SO2Cl, DIPEA, DCM, 3 h, RT; and (i) R3NCO/R3NCS, DCM, TEA, 4 h, RT.