Figure 1.
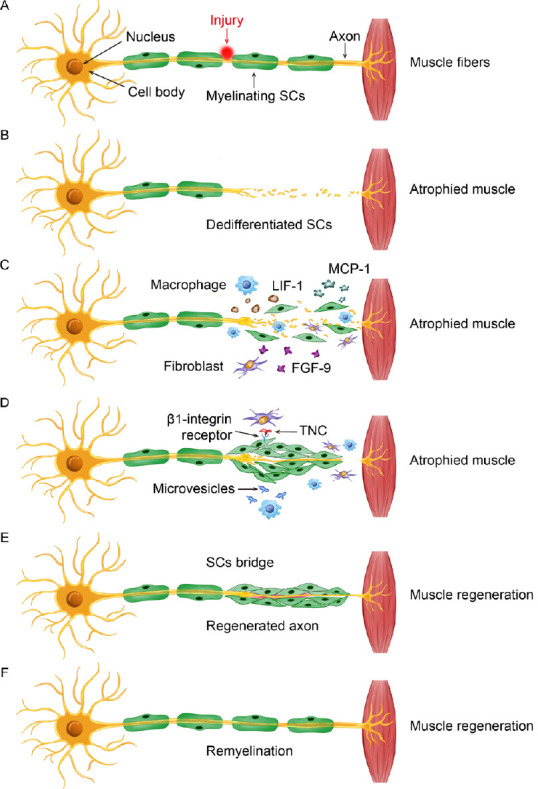
Schematic showing the general sequence of events following PNI involving fibroblasts, macrophages, and SCs to promote SC migration and nerve repair.
(A) Example of injury to a motor nerve projecting from the ventral horn of the spinal cord to target muscle tissue in the periphery. The cell body is located in the spinal cord, while the SC myelinated axon projects toward a target, where it synapses and initiates its action. (B) Nerve injury damages the integrity of the peripheral axon and induces Wallerian degeneration. (C) In response to injury, debris clearance and repair is initiated by activated SCs, recruited macrophages, and disrupted fibroblasts. Paracrine signaling involving MCP-1, LIF-1, and FGF-9 that is secreted from these cells facilitates a multicellular response to the injured nerve. (D) There is a fibroblast influence on SC behavior, which is partly mediated through direct binding of TNC to SC-expressed β1 integrin. Likewise, microvesicles released by activated macrophages also affect SC function in the repair process and depend on the inflammatory status of macrophages recruited to the injury site. (E) These cellular interactions contribute to the promotion of SC establishment of a bridge across the injury site for supporting axon regeneration and eventual myelination. (F) A repaired and regenerated peripheral nerve. FGF-9: Fibroblast growth factor 9; LIF-1: leukemia inhibitory factor-1; MCP-1: monocyte chemoattractant protein-1; PNI: peripheral nerve injury; SC: Schwann cell; TNC: tenascin-C.
