Figure 1. Conventional Myosin Structure and Nomenclature.
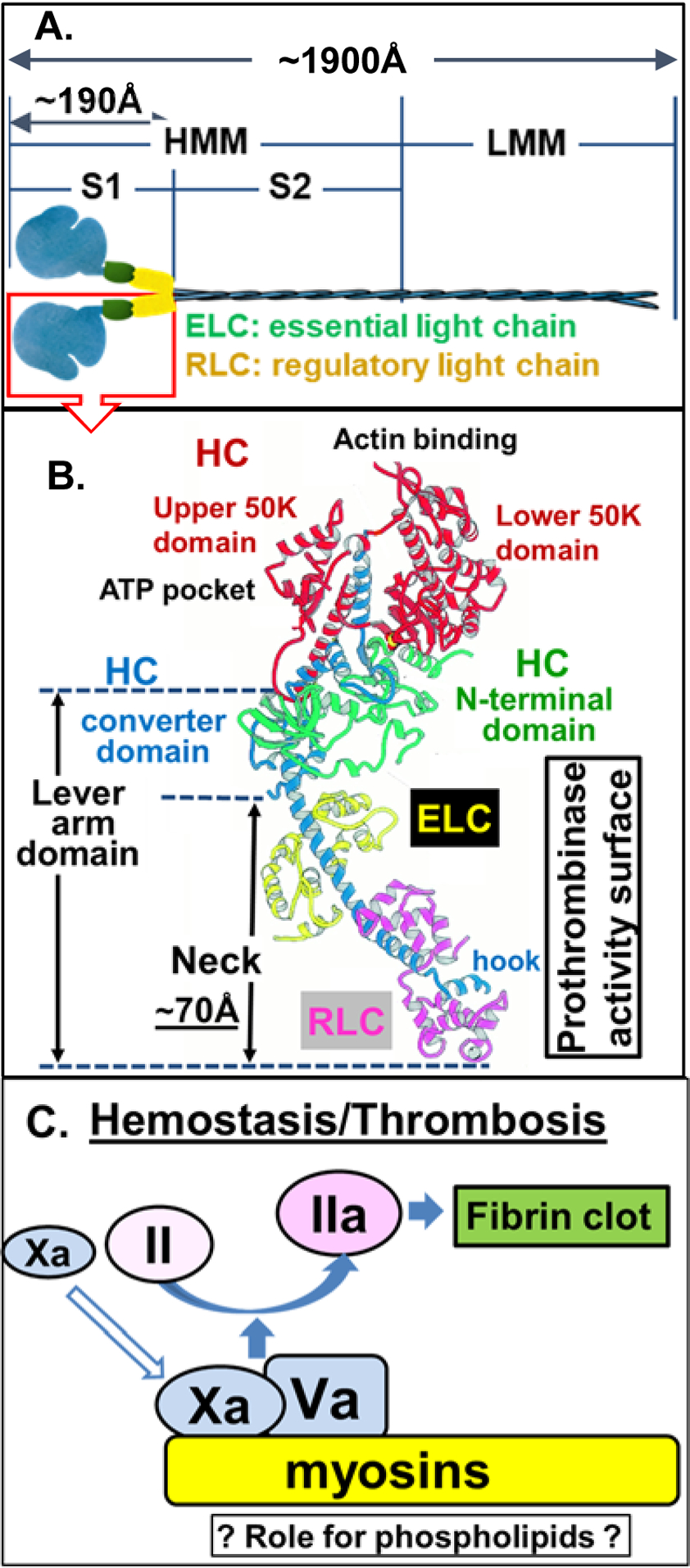
(A) Structure of conventional myosins which contain 6 polypeptides, 2 heavy chains (HC), and 4 light chains [2 ‘essential’ and 2 ‘regulatory’ light chains (ELC and RLC, respectively)]. Conventional myosin can be split into 1 light meromyosin (LMM) fragment and 1 heavy meromyosin (HMM) fragment; HMM can be further split into two subfragments, designated S1 and S2 subfragments [23]. (B) Structure of conventional myosin S1 domain containing a neck (N-terminal HC, ELC and RLC, the hypothesized procoagulant surface [24]), a converter domain (HC followed by neck), upper and lower 50 K domains that contain actin and ATP binding sites, in the N-terminal domain [19–22]. Structure shown in (B) is adopted with modifications from Rayment et al [21]. (C) Either SkM or CM (myosins) which is depicted on a yellow background can bind factors Xa and Va and thereby can enhance prothrombin activation on its surface. Kinetic studies indicate that the potency for SkM and CM preparations to enhance prothrombin activation is generally comparable to that of procoagulant phospholipid vesicles (Table 1). SkM preparations contain very low levels of phosphatidylserine [15], raising the queston of whether myosin-bound phospholipids may contribute to enhance SkM’s or CM’s procoagulant activity.
