Figure 1.
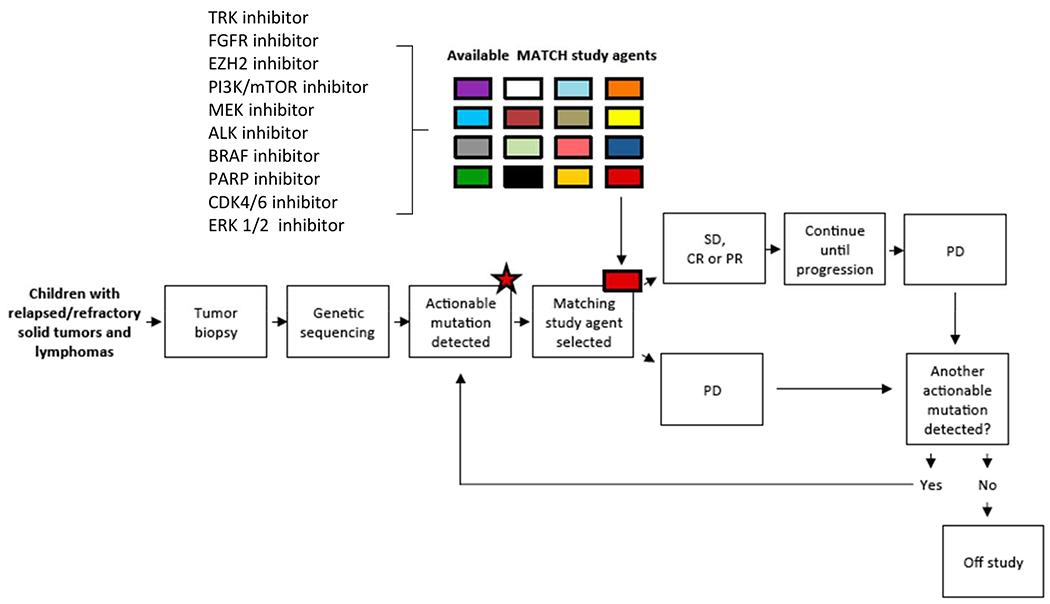
NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH) Trial Schema. TRK, tyrosine receptor kinase; FGFR, fibroblast growth factor receptor; EZH2, enhancer of zeste homolog 2; PI3K/mTOR, phosphoinositide 3-kinase/mammalian target of rapamycin; ALK, anaplastic lymphoma kinase; PARP, poly-ADP ribose polymerase; ERK, extracellular signal-regulated kinase; SD, stable disease; CR, complete response; PR, partial response; PD, progressive disease. From Allen CE, Laetsch TW, Mody R, et al. Target and Agent Prioritization for the Children’s Oncology Group-National Cancer Institute Pediatric MATCH Trial. J Natl Cancer Inst. 2017;109(5); with permission.
