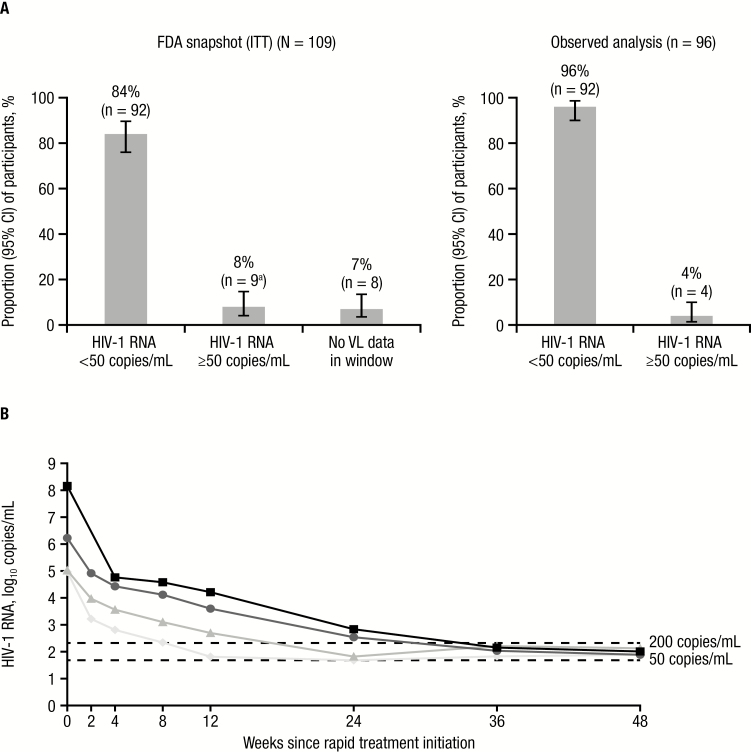
Figure 1.
Darunavir/cobicistat/emtricitabine/tenofovir alafenamide virologic efficacy in a rapid-initiation model of care. A, Virologic response at week 48. aThree participants discontinued early due to protocol-defined safety stopping rules. B, Log10 human immunodeficiency virus type 1 (HIV-1) RNA over time for individual participants with HIV-1 RNA ≥50 copies/mL at week 48 (observed analysis; n = 4). HIV-1 RNA level was not available for 1 participant at the week 2 visit. The participant with HIV-1 RNA 144 000 000 copies/mL at screening/baseline was a 30-year-old black/African American man with a CD4+ cell count of 242 cells/µL, Centers for Disease Control and Prevention (CDC) classification stage A, World Health Organization (WHO) clinical stage 1 (asymptomatic), and acute infection. The participant with HIV-1 RNA 1 680 000 copies/mL at screening/baseline was a 54-year-old white man with a CD4+ cell count of 8 cells/µL, CDC classification stage A, WHO clinical stage 1 (asymptomatic), and chronic infection. The participant with HIV-1 RNA 105 000 copies/mL at screening/baseline was a 28-year-old white man with a CD4+ cell count of 468 cells/µL, CDC classification stage A, WHO clinical stage 1 (asymptomatic), and early infection. The participant with HIV-1 RNA 92 900 copies/mL at screening/baseline was a 63-year-old black/African American woman with a CD4+ cell count of 127 cells/µL, CDC classification stage B, WHO clinical stage 2 (mild symptoms), and chronic infection. Abbreviations: CI, confidence interval; FDA, Food and Drug Administration; HIV-1, human immunodeficiency virus type 1; ITT, intent to treat; VL, viral load.