Figure 1.
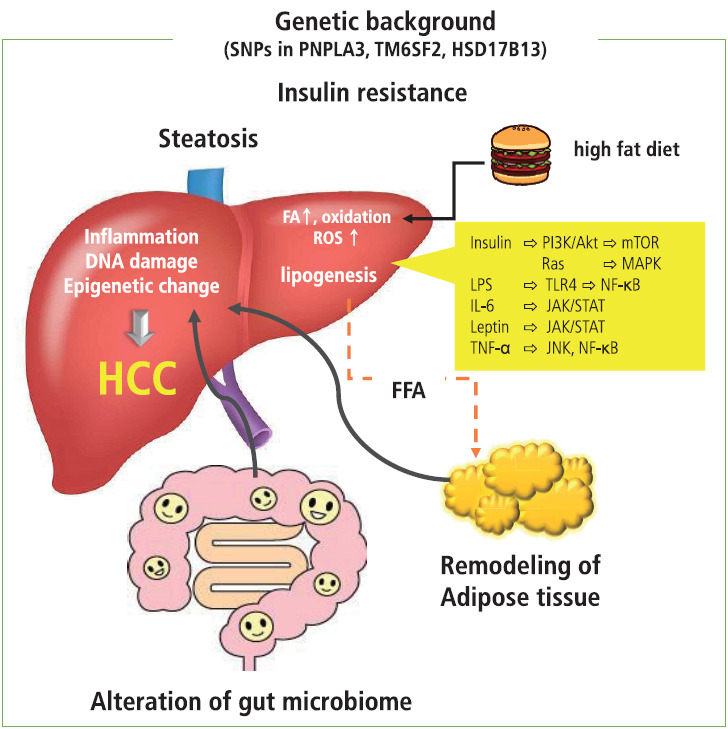
Remodeling of adipose tissue, alteration in gut microbiome, and increase in endoplasmic reticulum and oxidative stress act in concert and play a role in insulin resistance and establishment of NASH, which is a background condition of metabolic disease-related HCC. In patients with obesity, adipocyte inflammation and hypoxia take place, where proinflammatory cytokines and chemokines are prone to release. Under these conditions, insulin resistance induced by proinflammatory cytokines, adhesion molecules, and transcription factors contribute to the development of NASH. The excess amount of fatty acids induced by a high-fat diet results in the overload of oxidation in mitochondria, resulting in the generation of ROS that promote DNA damage. In addition, obesity-induced inflammation as well as a high-fat diet induce changes in the gut microbiome and increase permeability of the gut mucosa, leading to the leakage of inflammatory factors produced by the gut microbiome. These factors cause liver injury and DNA damage and eventually induce the progression of NASH and development of metabolic disease-related HCC through the activation of oncogenic pathways. Dietary factors and ROS induce epigenetic alterations that contribute to cell proliferation and carcinogenesis. In addition, the presence of several SNPs is associated with the development of NASH and HCC. It is possible that genetic factors associated with lipid metabolism and inflammation act as risk factors for metabolic disease-related HCCs. SNPs, single nucleotide polymorphisms; PNPLA3, patatin-like phospholipase domain containing 3; TM6SF2, transmembrane 6 superfamily member 2; HSD17B13, hydroxysteroid 17-beta dehydrogenase; HCC, hepatocellular carcinoma; FA, fatty acid; ROS, reactive oxygen species; FFA, free fatty acid; PI3K, phosphoinositide 3-kinase; mTOR, mammalian target of rapamycin; MAKP, mitogen activated protein kinase; LPS, lipopolysaccharide; TLR4, toll-like receptor 4; NF-κB, nuclear factor kappa B; IL-6, interleukin-6; JAK/STAT, Janus kinase/signal transducer and activator of transcription; TNF-α, tumor necrosis factor-α; JNK, Jun amino-terminal kinase.
