Figure 3.
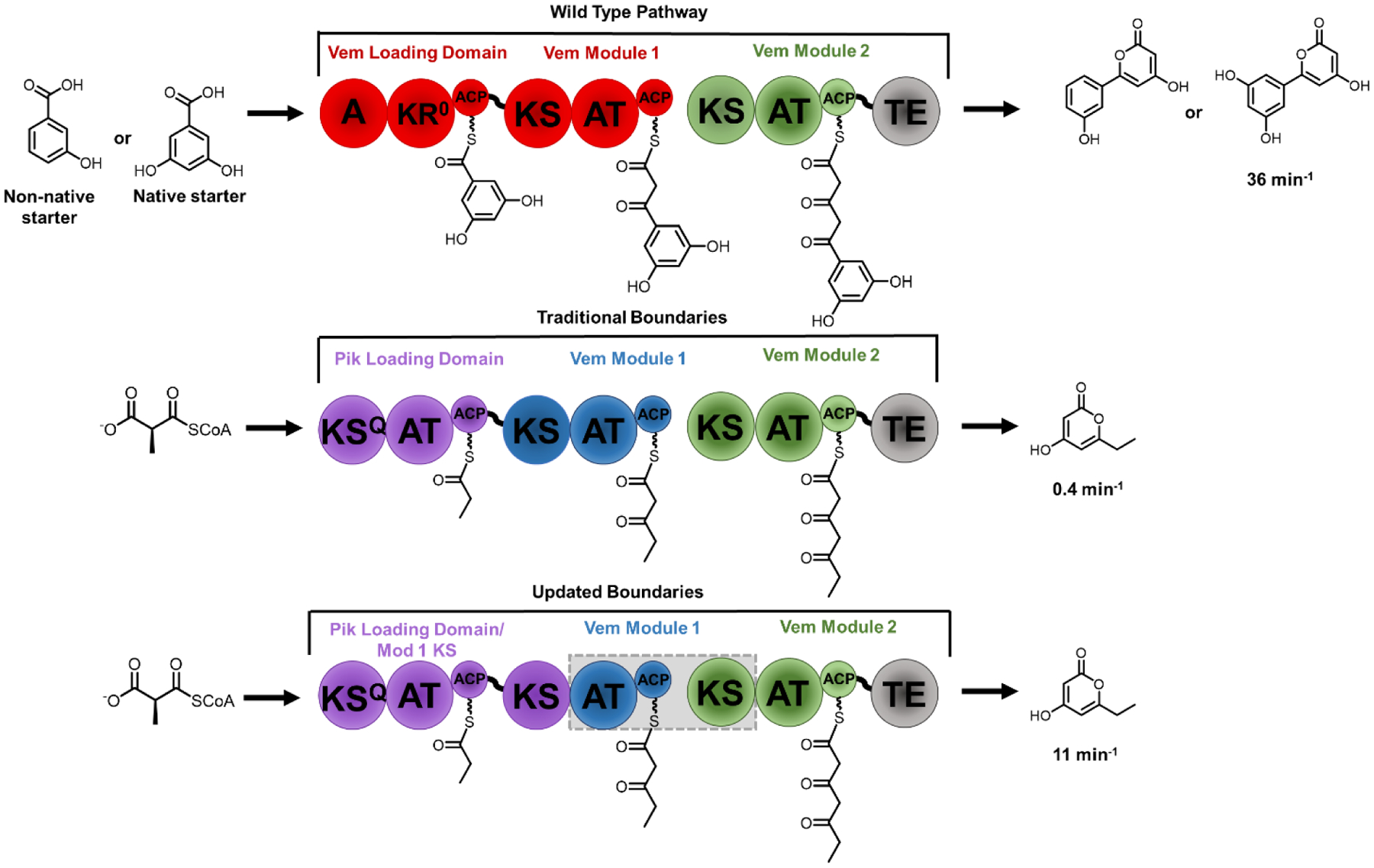
Hybrid synthases of the venemycin PKS (top) were constructed with traditional domain boundaries between the KS and ACP (middle). Module swaps were then constructed using boundaries between the KS and AT (gray box). The new boundary definition resulted in a chimera that was several-fold faster than its traditional boundary counterpart. A (adenylation domain), KR (Ketoreductase), ACP (acyl carrier protein), KS (ketosynthase), AT (acyltransferase), TE (thioesterase), KSQ (ketosynthase-like decarboxylase), KR0 (inactive ketoreductase).
