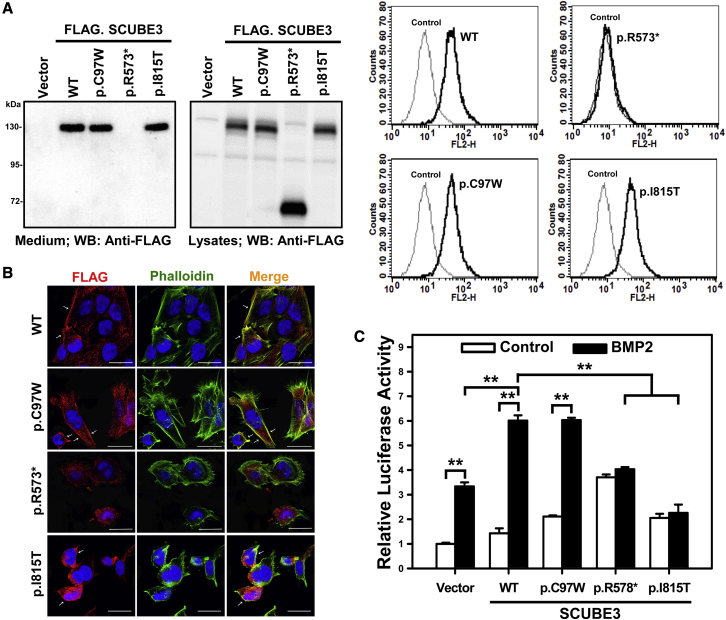
Figure 4.
Effect of disease-causing SCUBE3 variants on cell-surface protein levels and secretion and on BMP2 signaling
(A) SCUBE3 levels were analyzed in HEK293T cells. Samples from conditioned media and total cell lysates were collected and analyzed by western blot analysis using an anti-FLAG antibody (left panel). Empty vector-transfected cells were used as control. Representative blots from single experiments of three performed each are shown. A set of transfected cells was stained with anti-FLAG antibody and underwent flow cytometry (right panel).
(B) SAOS-2 cells were transfected with the expression plasmids encoding wild-type (WT) SCUBE3 and disease-associated variants (p.Cys97Trp, p.Arg573∗, and p.Ile815Thr). After 48 h, transfected cells were stained with mouse anti-FLAG antibody, Alexa Fluor 594 goat anti-mouse secondary antibody (red) and were analyzed by confocal microscopy. Alexa Fluor 488 phalloidin dye was used to stain the cortical actin associated with the plasma membrane (green). Nuclei are DAPI stained (blue). Merged images are shown in the right panels. White arrows indicate the distribution of the SCUBE3 proteins on the cellular surface in transfected cells. Red arrows indicate the dispersed localization of the p.Arg573∗ SCUBE3 variant in transfected cells. Scale bars represent 25 μm.
(C) HepG2 cells were transfected with the BMP-responsive luciferase reporter (BRE-luc) and pRL-TK alone or with the indicated expression plasmids. After 24 h, transfected cells were incubated for another 24 h with and without BMP2 (50 ng/mL), then luciferase activity was measured. Relative luciferase activity represents firefly luciferase values normalized to Renilla activity. The experiments were performed 3 times in triplicate. Data are mean ± SD. ∗∗p < 0.01.