Abstract
Aims
Adenosine receptors and extracellular adenosine have been demonstrated to modulate vascular smooth muscle cell (VSMC) proliferation and neointima formation. Adenosine kinase (ADK) is a major enzyme regulating intracellular adenosine levels but is function in VSMC remains unclear. Here, we investigated the role of ADK in vascular injury-induced smooth muscle proliferation and delineated the mechanisms underlying its action.
Methods and results
We found that ADK expression was higher in the neointima of injured vessels and in platelet-derived growth factor-treated VSMCs. Genetic and pharmacological inhibition of ADK was enough to attenuate arterial injury-induced neointima formation due to inhibition of VSMC proliferation. Mechanistically, using infinium methylation assays and bisulfite sequencing, we showed that ADK metabolized the intracellular adenosine and potentiated the transmethylation pathway, then induced the aberrant DNA hypermethylation. Pharmacological inhibition of aberrant DNA hypermethylation increased KLF4 expression and suppressed VSMC proliferation as well as the neointima formation. Importantly, in human femoral arteries, we observed increased ADK expression and DNA hypermethylation as well as decreased KLF4 expression in neointimal VSMCs of stenotic vessels suggesting that our findings in mice are relevant for human disease and may hold translational significance.
Conclusion
Our study unravels a novel mechanism by which ADK promotes VSMC proliferation via inducing aberrant DNA hypermethylation, thereby down-regulating KLF4 expression and promoting neointima formation. These findings advance the possibility of targeting ADK as an epigenetic modulator to combat vascular injury.
Keywords: Adenosine kinase, DNA methylation, Vascular smooth muscle cells, Arterial neointima
Graphical Abstract
1. Introduction
Vascular smooth muscle cells (VSMCs) are critical players in the development of a variety of arterial diseases including atherosclerosis, arterial restenosis, and hypertension.1–3 VSMCs are normally quiescent and contribute to the contractile force that enables blood vessels to regulate blood flow and pressure. However, in response to vascular injury, VSMCs in the media layer migrate into the intima, proliferate and produce a large amount of extracellular matrix. These phenotypic changes in VSMCs critically contribute to arterial neointima formation.4,5
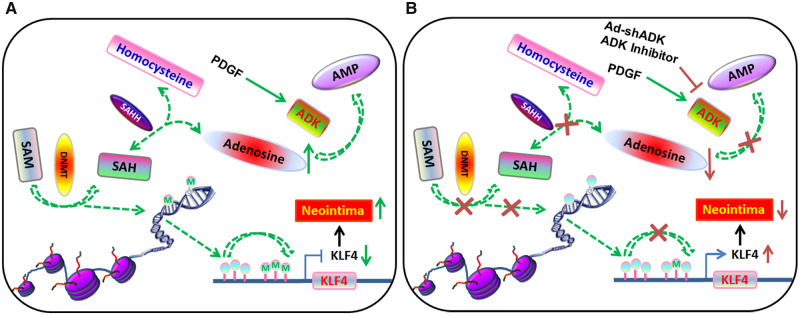
While many molecular pathways have been implicated in modulation of VSMC phenotypes and arterial neointima formation, recent studies have indicated the importance of epigenetic modification in these pathological changes.6,7 Among the known epigenetic mechanisms involved in vascular diseases, histone modification has been studied in depth,8,9 whereas only a few studies have focused on DNA methylation.10,11 DNA methylation, a process mediated by DNA methyltransferases (DNMTs), requires the donation of a methyl group from S-adenosylmethionine (SAM). S-adenosylhomocysteine (SAH), the resulting product from this transmethylation reaction, is further converted by SAH hydrolase (SAHH) into adenosine (Ado) and homocysteine (Hcy). The transmethylation pathway is controlled by mass action and the reversibility of the SAHH-mediated reaction. The equilibrium constant of the SAHH enzyme lies in the direction of SAH formation. Therefore, the reaction will proceed only when adenosine and homocysteine are constantly removed.12,13 In most tissues and cells, removal of adenosine is mainly through the enzyme adenosine kinase (ADK).12,13 When metabolic clearance of adenosine through ADK is impaired, the level of SAH rises and in turn, suppresses methyltransferase activity, reducing methylation of DNA (Supplementary material online, Figure S1A).13,14 Until now, the role of ADK in VSMC phenotypic determination has never been investigated, and it remains unknown whether the transmethylation pathway and DNA methylation regulated by ADK determines VSMC phenotype and pathological vascular remodelling associated with neointima formation following vascular injury.
In the current study, multiple approaches were employed, including the in vitro knockdown (KD) or overexpression of ADK in cultured VSMCs and the VSMC-selective deletion of Adk in vivo in novel mice, with the goal of interrogating the importance of ADK to the regulation of VSMC function and to address whether the intrinsic regulation of DNA methylation by targeting ADK and increasing adenosine alters VSMC behaviour in vascular remodelling. The results of this study will expand our understanding of vascular disease and help identify the therapeutic potential of targeting ADK for vascular remodelling in cardiovascular diseases.
2. Methods
2.1 Animal procedures
Animals were used based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocol was approved by the Institutional Animal Care and Use Committee at Augusta University. A Cre-loxP approach was used to generate VSMC-specific-Adk (Myh11-Cre/ERT2/Adkf/f) knockout mice. Details for the generation and breeding of Myh11-Cre/ERT2/Adkf/f knockout mice as well as Apoe−/−/Myh11-Cre/ERT2/Adkf/f mice are provided in the Supplementary material online, Methods and Materials. For generating models of carotid artery ligation and guide wire injury-induced arterial neointima formation, mice were anaesthetized using an intraperitoneal injection of ketamine (80 mg/kg body weight) and xylazine (5 mg/kg) (Phoenix Scientific, Inc., St. Joseph, MO, USA). For sacrificing mice, CO2 asphyxiation was used. Models of carotid artery ligation and guide wire injury-induced arterial neointima formation were performed as detailed in the Supplementary material online, Methods and Materials.
2.2 Molecular methods and reagents
The details of expression vectors, cell culture/transfection, isolation of mouse aortic SMCs, immunoblotting, RT–PCR, biochemical determinants of adenosine and SAH, flow cytometry, DNA bisulfite sequencing, haematoxylin and eosin (H&E) staining, and immunostaining methods are provided in the Supplementary material online, Methods and Materials. The WST-1 proliferation assay, DNMT activity assay and global DNA methylation assay were performed using commercial kits, the details of which are in the Supplementary material online, Methods and Materials. The Infinium methylation assay was analysed using Illumina Infinium 450K Methylation array according to the manufacturer’s suggested protocols (Illumina) and analysed as described in the Supplementary material online, Methods and Materials.
2.3 Human neointimal hyperplasia
All procedures involving human samples were carried out in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board at Augusta University. Informed consent was obtained from each participating patient. Human femoral artery specimens from femoral endarterectomy were collected and stained against ADK. ADK expression was compared between the medial and intimal layers.
2.4 Statistics
The minimum animal numbers and sample sizes required to achieve statistical significance were determined by power analysis and prior experience. Grouping was carried out in a randomized manner. The data were analysed with GraphPad Prism Software by one-way analysis of variance (ANOVA) with Tukey’s post hoc test, two-way ANOVA with Bonferroni’s post hoc test, or Student’s t-test to evaluate two-tailed levels of significance. The number of experiments performed is provided in figure legends (biological replicates). Values are expressed as the mean ± standard error of the mean and the null hypothesis was rejected at P ≤ 0.05.
3. Results
3.1 ADK expression is increased in proliferative VSMCs
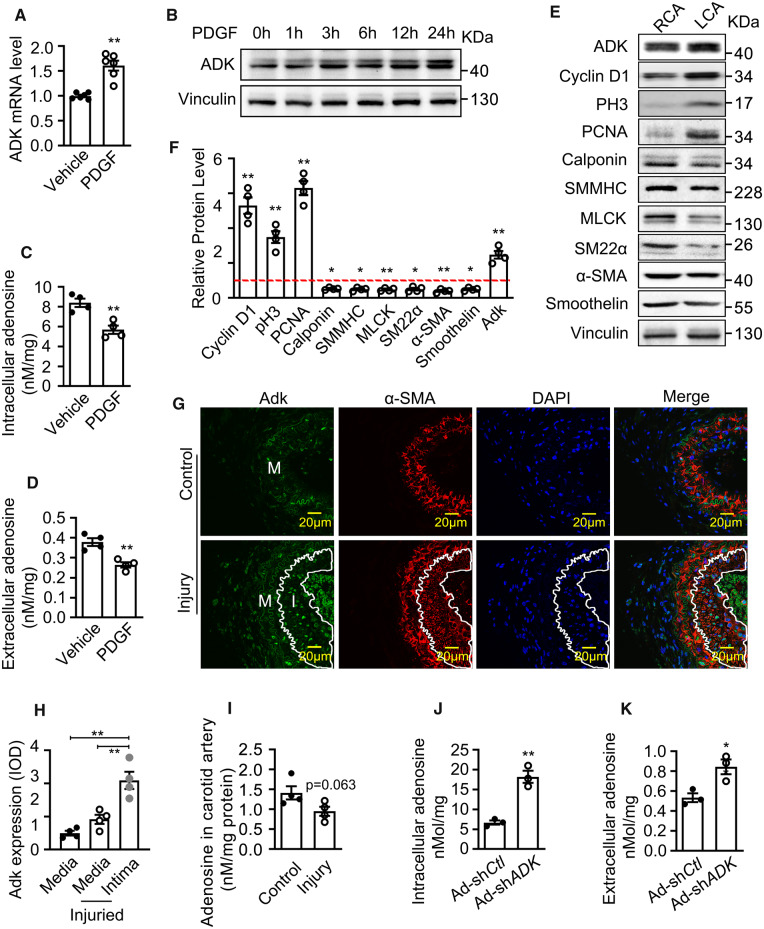
To examine ADK expression in proliferative VSMCs, cultured human coronary VSMCs (HCSMCs) were treated with platelet-derived growth factor (PDGF), which is a well-established mitogen to promote a switch of contractile VSMCs to proliferative cells.15 While moderate levels of ADK were detected in HCSMCs under control conditions, PDGF significantly up-regulated ADK expression at both the mRNA and protein levels, as shown by the results of quantitative RT–PCR, western blotting and immunostaining (Figure 1A and B, Supplementary material online, Figure S1B). The up-regulation of ADK had a functional impact on purine metabolism with the levels of both intracellular and extracellular adenosine significantly decreased in PDGF-treated HCSMCs (Figure 1C and D). Next, we examined the Adk expression and marks associated with VSMC proliferation in ligation-injured carotid arteries and found that, compared to control right carotid artery (RCA), the level of Adk was increased in the left carotid artery (LCA) 3 days after ligation, a mouse arterial injury model that resembles angioplasty in humans (Figure 1E and F). In addition, Adk expression was accompanied by increased expression of the proliferative markers cyclin D1, pH3 (phospho-histone H3), and PCNA (proliferating cell nuclear antigen), and down-regulation of the contractile SM markers calponin, SM MHC (myosin heavy chain), MLCK (myosin light-chain kinase), SM22α (smooth muscle protein 22-alpha), α-SMA (alpha-smooth muscle actin), and Smoothelin (Figure 1E and F). Immunostaining experiments revealed that the expression of Adk, which was primarily localized to the nuclei, was higher in VSMCs in the arterial neointima than those in the medial layer of ligated carotid arteries or in the vascular wall of intact mouse carotid arteries (Figure 1G and H). Nevertheless, the level of adenosine decreased in carotid arteries with neointima, although the decrease does not reach statistical significance (Figure 1I). To examine whether ADK regulates adenosine levels in VSMCs, ADK was silenced in HCSMCs using an adenovirus-delivered shRNA that reduced the ADK mRNA levels by 85% and protein levels by 51% (Supplementary material online, Figure S1C–E). The levels of intracellular and extracellular adenosine were enhanced by 200% and 50%, respectively, which suggest the decreased clearance of adenosine by ADK KD (Figure 1J and K).
Figure 1.
ADK expression and adenosine level in proliferative VSMCs. (A) Quantitative RT–PCR analysis of ADK mRNA levels in HCSMCs treated with vehicle or PDGF-BB (20 ng/mL) for 12 h (n = 6 independent cultures). (B) Representative western blots showing ADK expression in HCSMCs treated with vehicle or PDGF-BB (20 ng/mL) for time periods as indicated. Images are representative from three independent experiments. (C and D) Intracellular and extracellular adenosine levels of VSMCs. HCSMCs were treated with vehicle or PDGF-BB (20 ng/mL) for 24 h (n = 4 independent cultures). (E and F) Western blot detection (E) and densitometric quantification (F) of the indicated proteins normalized to Vinculin in HCSMCs treated with vehicle or PDGF-BB (20 ng/mL) for 48 h. Expression of proteins in vehicle-treated control group was set as 1 (red dashed line). Images are representative from four independent experiments. (G) Representative IF staining of Adk on sections of mouse carotid arteries from mice without or with ligation injury. Arterial neointima is indicated within the white line. (H) The Adk fluorescence relative optical density values in carotid arterial neointimal or medial layer from mice with ligation injury (n = 4 mice per group). (I) Quantification of adenosine levels in carotid arteries from mice without or with ligation injury (n = 4 mice per group). (J and K) Intracellular and extracellular adenosine levels in control and ADK KD HCSMCs (n = 3 independent cultures). For all bar graphs, data are expressed as the means ± SEM, *P < 0.05 and **P < 0.01 (unpaired, two-tailed Student’s t-test).
3.2 VSMC Adk ablation inhibits neointima formation in a model of vascular injury
To examine the effect of ADK on vascular remodelling, we selectively knocked out Adk in VSMCs in Myh11-Cre/ERT2/Adkf/f (hereinafter referred to as AdkΔVSMC) mice. These mice harbour a tamoxifen-inducible, VSMC-restricted null mutant in the Adk gene (Supplementary material online, Figure S2A and B) which, when activated by tamoxifen, results in the deletion of exon 7, yielding a kinase dead Adk gene that does not have the ability to metabolize adenosine. Western blot and immunostaining revealed a significant decrease in the level of Adk in the aortas and carotid arteries of tamoxifen-treated AdkΔVSMC mice vs. Adkf/f (hereinafter referred to as AdkWT) control mice (Supplementary material online, Figure S2C–E). In contrast, comparable levels of Adk expression were observed in other organs of tamoxifen-treated AdkΔVSMC mutants and control mice (Supplementary material online, Figure S2C). Consistent with these findings, marked attenuation of Adk protein and mRNA levels were observed in VSMCs isolated from the aortas of tamoxifen-treated AdkΔVSMC mice compared with AdkWT control mice (Supplementary material online, Figure S2F and G). Accordingly, the levels of adenosine in aortas of AdkΔVSMC mice were much higher than those of AdkWT mice (Supplementary material online, Figure S2H). No gross morphological differences were observed on H&E-stained cross-sections of carotid artery (Supplementary material online, Figure S2I) and thoracic artery (Supplementary material online, Figure S2J) from AdkWT vs. AdkΔVSMC mice.
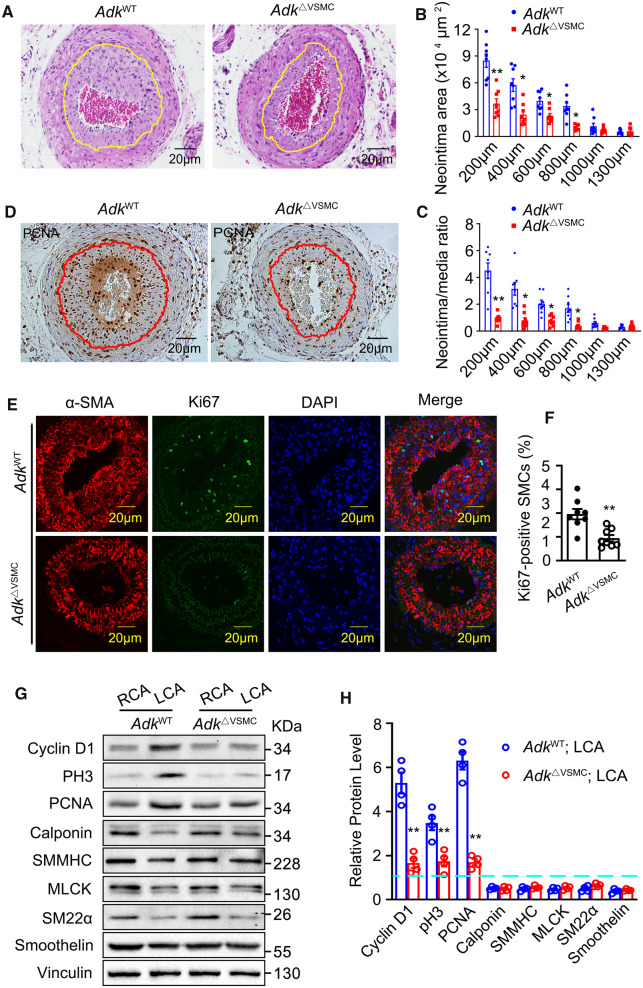
To determine whether the loss of Adk in VSMCs affects arterial neointima formation, the left common carotid arteries were ligated for 28 days and then collected for histological analysis. Quantification of the neointima area and neointima/media ratio based on serial cross-sections of carotid arteries with relative distances to the ligation site showed decreased vascular remodelling in AdkΔVSMC mice compared with AdkWT mice (Figure2A–C). In addition, the number of PCNA- or Ki67-positive VSMCs in cross-sections of arterial neointima was greatly reduced in AdkΔVSMC mice compared with AdkWT mice (Figure 2D–F). To identify the role of ADK in VSMC phenotypic switching, we analysed the expression levels of proliferative and contractile markers in RCAs and LCAs harvested from AdkWT and AdkΔVSMC mice 3 days post-ligation. We found that, compared with control AdkWT mice, VSMC-specific Adk deletion significantly attenuated the induction of proliferative marker cyclin D1, pH3, and PCNA in ligated LCA. However, Adk deletion did not rescue the ligation injury-induced down-regulation of multiple contractile markers in LCA (Figure 2G and H). Collectively, these data imply that the reduced neointima formation in mice deficient in VSMC Adk is due to changes in cell proliferation.
Figure 2.
Neointima formation and VSMC proliferation in AdkWT and AdkΔVSMC mice following carotid artery ligation. (A) Representative H&E-stained cross-sections of carotid arteries from mice with left common carotid artery ligation injury for 28 days. Yellow lines indicate the internal elastic lamina. (B) Quantitative analysis of arterial neointima area (n = 8 mice per group). (C) Calculation of the ratios of neointima areas to medial areas of injured carotid arteries (n = 8 mice per group). (D) Representative IHC staining of PCNA on sections of ligated carotid arteries. Red lines indicate the internal elastic lamina. (E) Representative IF staining of Ki67 on sections of ligated carotid arteries. (F) Quantification of Ki67-positive VSMCs in neointima (n = 8 mice per group). (G and H) Western blot detection (G) and densitometric quantification (H) of the indicated proteins normalized to Vinculin in the injured left (LCA) and uninjured right carotid arteries (RCA) of AdkWT and AdkVEC-KO mice 72 h post-ligation injury (n = 4 mice per group). Expression of proteins in the RCA of the AdkWT group was set as 1 (blue dashed line). For all bar graphs, data are expressed as the means ± SEM, *P < 0.05 and **P < 0.01 (unpaired, two-tailed Student’s t-test).
3.3 ADK KD/inhibition suppresses VSMC proliferation in a cell-autonomous manner
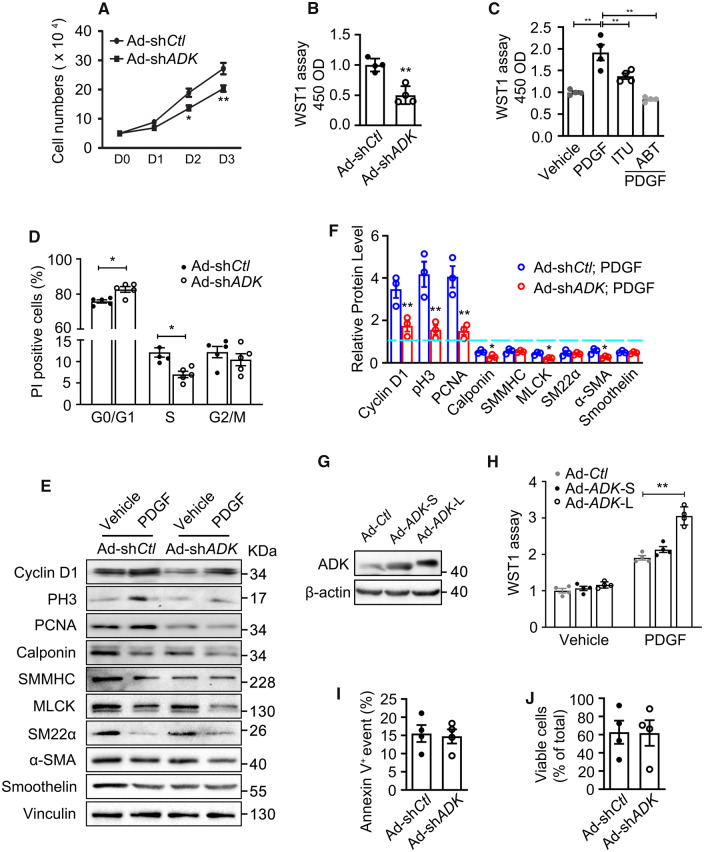
To further investigate the effect of ADK KD on VSMC proliferation, cell counting, and WST-1 proliferation assays were performed. Compared with control cells, the growth of ADK KD HCSMCs was significantly decreased (Figure 3A and B). In an alternative approach to ADK KD, the pharmacological inhibition of ADK with 5-iodotubercidin (ITU, 10 µM for 24 h) and 5-(3-Bromophenyl)-7-[6-(4-morpholinyl)-3-pyrido[2,3-d]byrimidin-4-amine dihydrochloride (ABT702, 10 µM for 24 h) also inhibited PDGF-induced growth of HCSMCs as determined by the WST assay (Figure 3C). Flow cytometric analysis of the cell cycle by DNA staining with propidium iodide (PI) found that VSMCs deficient in ADK were most commonly in the G0/G1 phase and a lower percentage in the S phase, indicating that loss of ADK expression leads to cell cycle arrest at G0/G1 (Figure 3D). Consistent with the above decreased proliferative phenotype, western blotting showed that the increased levels of cyclin D1, pH3, and PCNA induced by PDGF treatment were markedly decreased in ADK KD VSMCs when compared with control VSMCs (Figure 3E and F). To further confirm that ADK regulates VSMC proliferation, we respectively overexpressed the two alternatively spliced isoforms, ADK long (ADK-L) and ADK short (ADK-S) in HCSMCs (Figure 3G). The WST assay showed that overexpression of each isoform of ADK had a minimal effect on HCSMC proliferation; however, overexpression of ADK-L significantly potentiated the PDGF-induced proliferation of HCSMCs (Figure 3H).
Figure 3.
Proliferation and apoptosis in control and ADK KD VSMCs. (A) Cell numbers of ADK KD and control HCSMCs grown in complete growth medium were counted for three consecutive days (n = 3 independent experiments). (B) WST-1 proliferation analysis of HCSMCs that were transfected with ADK KD and control adenovirus and grown in complete growth medium for 48 h (n = 4 independent experiments). (C) WST-1 proliferation analysis of vehicle or PDGF-treated HCSMCs pretreated with ADK inhibitors 5-iodotubercidin (ITU, 10 µM) or 5-(3-Bromophenyl)-7-[6-(4-morpholinyl)-3-pyrido [2,3-d] byrimidin-4-amine dihydrochloride (ABT702, 10 µM) (n = 4 independent experiments). (D) Flow cytometry analyses of cell cycle for control and ADK KD HCSMCs grown in complete growth medium with propidium iodide (PI) DNA staining (n = 5 independent experiments). (E and F) Western blot detection (E) and densitometric quantification (F) of the indicated proteins normalized to Vinculin in control and ADK KD HCSMCs treated with vehicle or PDGF-BB (20 ng/mL) for 48 h (n = 3 independent experiments). Expression of proteins in vehicle-treated control group was set as 1 (blue dashed line). (G) Western blot analysis of ADK in HCSMCs transfected with the cytoplasmic (ADK-S) or nuclear (ADK-L) isoform of ADK. Images are representative from three independent experiments. (H) WST-1 proliferation analysis of vehicle or PDGF-treated HCSMCs transfected with the ADK-S and ADK-L isoforms (n = 4 independent experiments). (I) Flow cytometry analyses of apoptosis for control and ADK KD HCSMCs grown in complete growth medium with Annexin V staining (n = 4 independent experiments). (J) Cell viability for control and ADK KD HCSMCs grown in complete growth medium was evaluated by counting the relative number of cells that excluded trypan blue dye (n = 4 independent experiments). For all bar graphs, data are expressed as the means ± SEM, *P < 0.05 and **P < 0.01 (two-way ANOVA with Bonferroni’s post hoc test for A; one-way ANOVA with Tukey’s post hoc test for C and F; unpaired, two-tailed Student’s t-test for B, D, and H–J).
We next investigated whether reduced cell growth in ADK KD VSMCs is associated with apoptosis. The degree of apoptosis was assessed by flow cytometry of ADK KD or control HCSMCs. As shown in Figure 3I, no differences in VSMC apoptosis were observed between ADK KD and the control group. There was also no difference in cell necrosis between these two groups as determined by trypan blue exclusion (Figure 3J). These data indicate that there is no overt change in apoptosis or necrosis in cells with the loss of ADK expression.
3.4 VSMC DNA hypermethylation correlates with ADK overexpression and neointimal hyperplasia
Previous studies have demonstrated that adenosine inhibits the proliferation of murine and human VSMCs via activation of the A2BR receptor.16,17 We found that genetic or pharmacological inactivation of A2BR only modestly augmented the proliferation potential of HCSMCs (Supplementary material online, Figure S3A–C). Furthermore, the anti-proliferative effect of ADK deletion in HCSMCs was not reversed by inactivation of A2BR (Supplementary material online, Figure S3A–C). This data suggest that a mechanism other than A2BR mediates the effect of ADK deletion on VSMC proliferation.
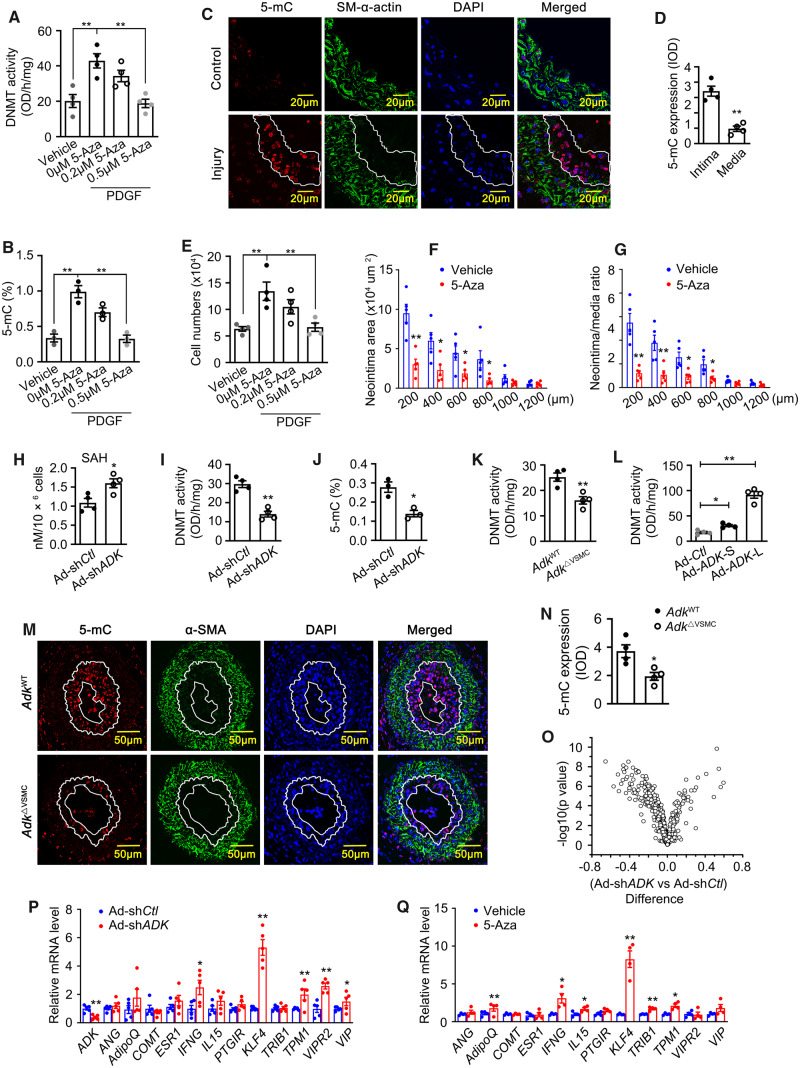
Elevated intracellular adenosine has been shown to shift the equilibrium of the SAHH-mediated reaction towards the accumulation of SAH, which is a powerful inhibitor of SAM-dependent transmethylation reactions.14 Furthermore, a form of ADK that is localized to the nucleus has been demonstrated to sustain DNA methylation.13 In light of the nuclear localization of Adk observed in neointimal VSMCs, we hypothesized that elevated levels of intracellular adenosine triggered by Adk deletion inhibits VSMC proliferation and neointima formation through the reduced methylation of DNA. To test this hypothesis, we first examined whether DNA hypermethylation is associated with VSMC proliferation and neointima formation. Along with an increase in ADK expression (Figure 1A and B), the activity of DNMT and the level of 5-methylcytidine (5-mC, an indicator of global DNA methylation) were increased in PDGF-treated VSMCs (Figure 4A and B). Immunohistochemical (IHC) staining of ligated carotid arteries revealed greater increases in 5-mC levels in neointimal vs. medial VSMCs, which was consistent with previous changes in Adk expression (Figures 4C and D and 1E). The spatial match between ADK overexpression and increased 5-mC immunoreactivity suggests that a functional relationship may exist between ADK, methyltransferase activity and the degree of DNA methylation. In addition, these data also suggest that DNA hypermethylation correlates with, and might contribute to, enhanced VSMC proliferation and neointimal hyperplasia.
Figure 4.
Involvement of ADK-driven DNA hypermethylation in VSMC proliferation and neointima formation. Quantification of DNA methyltransferase (DNMT) activity (A) and 5-methylcytosine (5-mC) content (B) in HCSMCs pretreated with 5-Aza (2 μmol/L or 5 μmol/L) for 1 h and followed by incubation with PDGF (20 ng/mL) for 72 h (n = 4 for A and 3 for B). Representative IF staining (C) and quantification (D) of 5-mC on sections of mouse carotid arteries from mice without or with ligation injury (n = 4 mice per group). Arterial neointima is indicated within the white line. (E) WST-1 proliferation analysis of HCSMCs pretreated with 5-Aza-2′-deoxycytidine (5-Aza, 2 μmol/L or 5 μmol/L) for 1 h followed by incubation with PDGF (20 ng/mL) for 72 h (n = 4). Quantitative analysis of arterial neointima area (F) and ratios of neointima areas to medial areas of injured carotid arteries (G) from 5-Aza-treated mice (n = 5 mice per group). Quantification of SAH level (H), DNMT activity (I), and 5-mC content (J) in control and ADK KD HCSMCs growing in complete medium (n = 3 or 4 independent experiments). Quantification of DNMT activity (K) in mouse aortic SMCs isolated from AdkWT or AdkΔVSMC mice (n = 4 independent experiments). (L) Quantification of DNMT activity in HCSMCs transfected with the ADK-S and ADK-L isoforms (n = 4 independent experiments). Representative IF staining (M) and quantification of 5-mC (N) on sections of mouse carotid arteries from AdkWT or AdkΔVSMC mice with ligation injury. The area highlighted with the white line is the arterial neointima (n = 4 mice per group). (O) Mean methylation difference of CpG sites in the promoters of 30 anti-proliferative genes of VSMCs comparing control group with those of ADK KD HCSMCs. n = 3 independent cultures, x axis: mean methylation difference of CpG sites between control and ADK KD HCSMCs; y axis: −log10 of the P-values. (P) Quantitative RT–PCR analysis of mRNA levels for anti-proliferative genes with DNA hypomethylation in the promoters due to ADK KD in control and ADK KD HCSMCs (n = 6 independent experiments). (Q) Quantitative RT–PCR analysis of mRNA levels for genes in (H) in HCSMCs treated with 5 μmol/L 5-Aza or vehicle for 72 h (n = 4 or 5 independent experiments). For all bar graphs, data are expressed as the means ± SEM, *P < 0.05 and **P < 0.01 (one-way ANOVA with Tukey’s post hoc test for A, B, E, and L; unpaired, two-tailed Student’s t-test for D, F–K, and N–Q).
3.5 Inhibition of DNA methylation suppresses VSMC proliferation and neointimal hyperplasia
To determine whether changes in DNA methylation can contribute to the proliferative phenotype of VSMCs, a dose–response study with the DNMT inhibitor 5-Aza was performed. As shown in Figure 4A, B and E, administration of 5-Aza for 3 days significantly reduced DNMT activity, 5-mC content and the cell numbers of HCSMCs in a dose-dependent manner. To determine whether changes in DNA methylation impact neointima formation in vivo, we treated C57BL/6 mice with 5-Aza at 0.2 mg/kg/day for 5 weeks following carotid artery ligation. As shown in Figure 4F and G, administration of 5-Aza significantly reduced the neointima area and the neointima/media ratio. These data suggest that inhibition of DNMT activity and DNA methylation suppresses VSMC proliferation and reduces neointima formation.
3.6 ADK determines DNA methylation level in VSMCs
To investigate whether ADK deletion alters DNA methylation in VSMCs, we next examined the level of SAH and activity of DNMTs in VSMCs. ADK KD markedly enhanced the level of intracellular SAH and reduced DNMT activity in HCSMCs (Figure 4H and I). Consistent with these results, the levels of 5-mC in ADK KD HCSMCs were much lower than those in control HCSMCs (Figure 4J). Similar changes in DNMT activity and 5-mC levels were observed in arterial VSMCs isolated from AdkΔVSMC and AdkWT mice (Figure 4K and Supplementary material online, Figure S3D). In addition, gain-of-function studies demonstrated that both ADK isoforms increased the DNMT activity and 5-mC levels in HCSMCs, and the increase was more prominent with ADK-L (Figure 4L and Supplementary material online, Figure S3E). IHC staining showed that 5-mC immunoreactivity was significantly decreased in the neointimal VSMCs from AdkΔVSMC mice compared with AdkWT mice (Figure 4M and N). In HCSMCs, ADK KD-induced DNA hypomethylation was preserved following KD of the A2AR or A2BR (Supplementary material online, Figure S3F–H), indicating that activation of the key adenosine receptors is not required for the reduction in DNA methylation seen with ADK KD HCSMCs. Collectively, these data provide evidence of a functional link between ADK activity and DNA methylation status.
To further validate the link between ADK KD and DNA hypomethylation and to determine which genes are regulated by promoter hypomethylation, we next performed the Infinium methylation assay. This genome-wide DNA methylation assay includes at least 485 000 methylation sites covering 96% of the CpG islands distributed across the promoter, gene body, and 3′UTR of 99% of Ref Seq genes. Since promoter hypermethylation correlates with gene silencing, we restricted the analysis to probes within promoter regions. For a specific CpG site, methylation differences were calculated by subtracting the mean methylation value in the control group from the corresponding value in the ADK deletion group. As defined in the programme of Gene Set Enrichment Analysis (GSEA), the analysis of methylation differences was confined to a group of 30 genes that negatively regulate proliferation of VSMCs (Supplementary material online, Table S1). Methylation on most of the CpG sites for the 30 gene promoters were decreased in HCSMCs deficient in ADK compared with the controls, as shown in the volcano plot in Figure 4O. Based on the statistical analysis of false discovery rates (FDRs) <0.2, the methylation levels of 16 CpG sites that mapped to 12 anti-proliferative genes in the ADK KD group were considered to be significantly decreased (highlighted in Supplementary material online, Table S1). Quantitative RT–PCR was performed to examine the levels of mRNA for those 12 genes with decreased methylation in their promoters. Increased levels of mRNA were found for the anti-proliferative genes INFG, Krüppel-Like Factor 4 (KLF4), TPM1, VIPR2, and VIP in ADK-deficient HCSMCs (Figure 4P). We also examined the mRNA levels of these 12 genes in 5-Aza-treated HCSMCs. Administration of 5-Aza for 3 days significantly up-regulated the mRNA levels of AdipoQ, INFG, IL-15, KLF4, TRIB1, and TPM1 in HCSMCs (Figure 4Q). From these two sets of RT–PCR data, we found that the greatest up-regulation in gene expression was observed with KLF4, which is a key suppressor of VSMC proliferation.18–22 We interrogated a microarray data set derived from ADK KD and control human umbilical vein endothelial cells and found that KLF4 was also increased at the mRNA level in ADK KD endothelial cells. These data suggest that KLF4 up-regulation might at least partially contribute to the changes in VSMC proliferation and neointima formation seen with ADK KD VSMCs.
3.7 Decreased proliferation in ADK KD VSMCs is causally associated with up-regulated KLF4
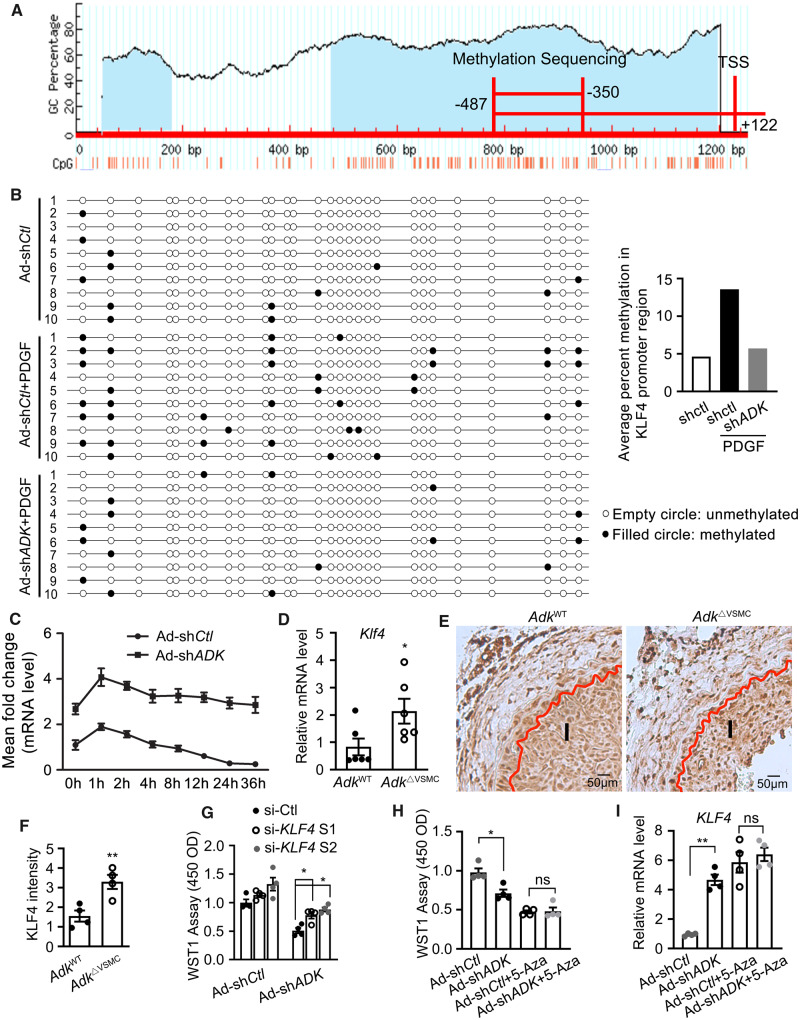
To validate the data from the Infinium methylation assay, changes in the DNA methylation of the KLF4 promoter were assessed by bisulfite sequencing. A CpG island predicated by Methyl Primer Express software exists between −2000 bp and +200 bp at the transcription start site (TSS). Bisulfite sequencing of KLF4 was conducted within −487 bp to +122 bp of the TSS (Figure 5A). Methylation within the fragment between −478 bp and −350 bp of the TSS was significantly altered; the methylation rates of CpG sites were 12.86% and 5.36%, respectively, for control and ADK KD HCSMCs with PDGF treatment (Figure 5B). These data indicate that ADK deletion dramatically decreases the methylation of DNA in the KLF4 promoter region. Furthermore, we also found that prolonged PDGF treatment (3 days) increased the degree of methylation of CpG sites between −478 bp and −350 bp of the TSS for KLF4 by one fold (Figure 5B). Consistent with the increased methylation status in the KLF4 promoter, mRNA levels of KLF4 were down-regulated in HCSMCs with prolonged PDGF treatment (Figure 5C). However, in ADK KD cells, the down-regulation of KLF4 expression was prevented (Figure 5C). All together, these data suggest that PDGF treatment leads to pathological increases in ADK expression that down-regulate KLF4 by stimulating DNA hypermethylation.
Figure 5.
The involvement of KLF4 up-regulation due to DNA hypomethylation in ADK deficiency-induced suppression of VSMC proliferation. (A) Schematic illustration of the predicted CpG islands within promoter region of the KLF4 gene (http://www.urogene.org/methprimer/). (B) DNA bisulfite analysis of the 137 bp region (−478 bp to −350 bp of TSS) within the KLF4 promoter of control and ADK KD HCSMCs treated with 20 ng/mL PDGF-BB or vehicle for 72 h (n = 10). (C) Quantitative RT–PCR analysis of mRNA level of KLF4 in ADK KD and control HCSMCs treated with 20 ng/mL PDGF-BB for indicated time points (n = 4 independent experiments). (D) Quantitative RT–PCR analysis of KLF4 transcription level in mouse aortic SMCs isolated from AdkWT and AdkΔVSMC mice (n = 6 independent experiments). (E and F) Representative IHC staining of KLF4 expression and quantification in ligated carotid arteries of AdkWT or AdkΔVSMC mice with ligation injury for 14 days. I indicates intimal area (n = 4 mice per group). (G) Analysis of proliferation by WST-1 assay in HCSMCs that were first transfected with siRNA targeting KLF4 or control siRNA, and then infected 6 h later with adenovirus encoding shRNA targeting ADK or control shRNA (n = 4 independent experiments). (H) Analysis of proliferation by WST-1 assay in HCSMCs that were first infected with adenovirus encoding shRNA targeting ADK or control shRNA, and then treated 6 h later with 5-Aza at 2 μM for 48 h (n = 4). (I) Quantitative RT–PCR analysis of KLF4 transcription level in HCSMCs that were first infected with adenovirus encoding shRNA targeting ADK or control shRNA, and then treated 6 h later with 5-Aza at 2 μM for 48 h (n = 4). For all bar graphs, data are expressed as the means ± SEM, *P < 0.05 and **P < 0.01 (two-way ANOVA with Bonferroni’s post hoc test for C; unpaired, two-tailed Student’s t-test for D and F; one-way ANOVA with Tukey’s post hoc test for G–I).
To establish a link between ADK levels, KLF4 levels and vascular remodelling, we examined the Klf4 expression in carotid arteries of AdkΔVSMC and AdkWT mice. Increased expression of Klf4 was found in arteries of AdkΔVSMC mice at both the mRNA and protein levels (Figure 5D–F). To determine the importance of KLF4 up-regulation to the reduced proliferative capacity of ADK KD VSMCs, two siRNAs that respectively lowered the mRNA level of KLF4 by 58% and 66% were introduced. KLF4 KD partially, but significantly, impaired the ability of ADK KD to suppress VSMC proliferation (Figure 5G).
To further confirm that the reduced proliferation of VSMCs is a direct consequence of ADK KD-induced hypomethylation of the KLF4 promoter, HCSMCs were first treated with the DNMT inhibitor 5-Aza, followed by treatment with adenoviral ADK shRNA. As shown previously (Figure 4E and Q), 5-Aza treatment impaired the proliferative ability of HCSMCs and increased the mRNA level of KLF4, but the loss of ADK expression did not further decrease proliferation or elevate the mRNA level of KLF4 (Figure 5H and I), suggesting that the effect of ADK KD on KLF4 expression and VSMC proliferation cannot be achieved in VSMCs with hypomethylated genes.
3.8 Adk deletion reduces guide wire injury-induced arterial neointima formation in atherosclerotic mice
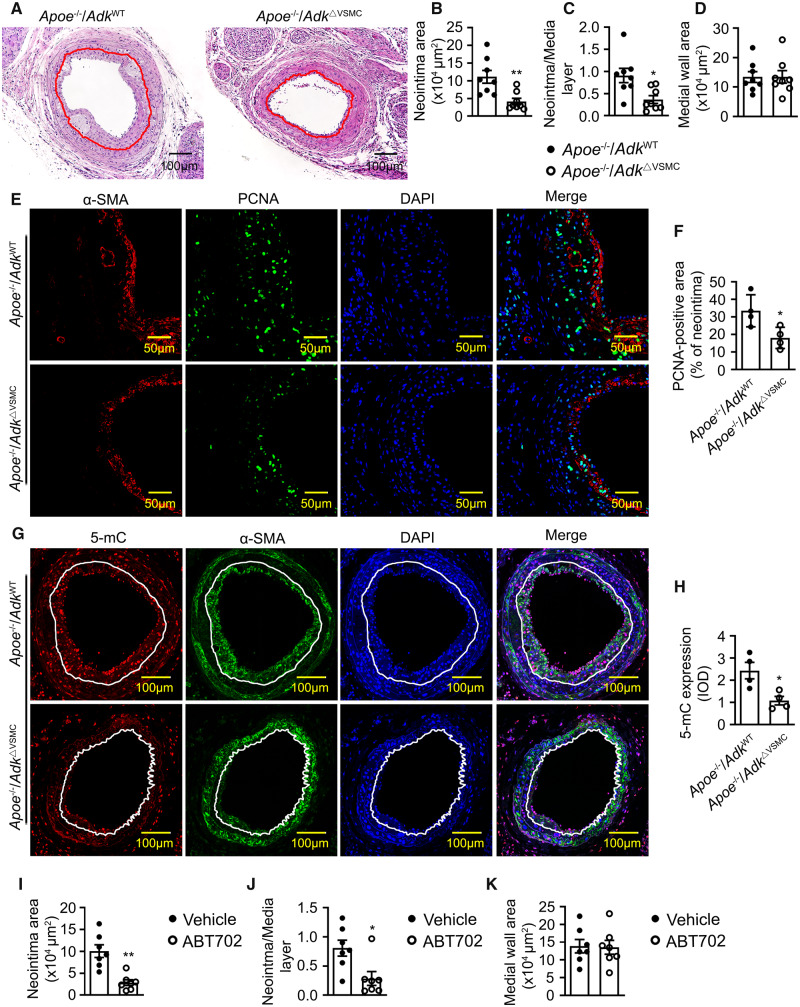
To determine whether the selective deletion of Adk in VSMCs reduces the arterial neointima in a disease model analogous to human arterial restenosis, AdkΔVSMC mice and controls were bred to hypercholesterolaemia Apoe−/− mice, and a guide wire transluminal injury in carotid arteries was performed. Three weeks following arterial injury, a markedly decreased arterial neointima was found in Apoe−/−/AdkΔVSMC mice compared with that in control mice (Figure 6A–C). No obvious difference in the medial wall area was observed between Apoe−/−/AdkΔVSMC and Apoe−/−/AdkWT groups (Figure 6D), which is consistent with the non-apoptotic/necrotic effects of ADK KD in vitro. As shown in Figure 6E and F, the number of PCNA-positive VSMCs is greatly reduced in the arterial vessel wall of Apoe−/−/AdkΔVSMC mice compared with control mice. In addition, Adk deletion in VSMCs also significantly decreased the 5-mC content in carotid arterial neointima of Apoe−/− mice following a guide wire transluminal injury (Figure 6G and H). Collectively, these data indicate that Adk deletion in VSMCs ameliorates vascular injury-induced arterial neointima formation by reducing aberrant DNA hypermethylation and excessive VSMC proliferation.
Figure 6.
The role of ADK in guide wire injury-induced arterial neointima formation in atherosclerotic mice. (A) Representative H&E-stained cross-sections of mouse carotid arteries from AdkWT and AdkΔVSMC mice with guide wire injury for 21 days. Red lines indicate the internal elastic lamina. (B) Quantitative analysis of arterial neointima area in A (n = 8 mice per group). (C) Quantification of the ratios of neointima areas to media areas of injured carotid arteries in A (n = 8 mice per group). (D) Quantitative analysis of arterial media area in A (n = 8 mice per group). (E) Representative IF staining of PCNA on sections of carotid arteries from Apoe−/−/AdkWT and Apoe−/−/AdkΔVSMC mice with guide wire injury for 21 days. (F) Quantification of PCNA-positive area in carotid arterial neointima from Apoe−/−/AdkWT and Apoe−/−/AdkΔVSMC mice with guide wire injury for 21 days (n = 4 mice per group). (G) Representative IF staining of 5-mC on sections of mouse carotid arteries from Apoe−/−/AdkWT or Apoe−/−/AdkΔVSMC mice with guide wire injury for 21 days. (H) The 5-mC fluorescence relative optical density values in carotid arterial neointima from Apoe−/−/AdkWT or Apoe−/−/AdkΔVSMC mice with guide wire injury for 21 days (n = 4 mice per group). (I–K) Evaluation of neointima formation in carotid arteries from mice with left common carotid artery ligation injury. Mice were injected intraperitoneally with vehicle or ABT702 at a dose of 1.5 mg/kg mouse/day for 21 days (n = 7 mice per group). (I) Quantitative analysis of arterial neointima area. (J) Quantification of the ratios of neointima areas to media areas of injured carotid arteries. (K) Quantitative analysis of arterial media area. For all bar graphs, data are expressed as the means ± SEM, *P < 0.05 and **P < 0.01 (unpaired, two-tailed Student’s t-test).
To assess the therapeutic potential of pharmacological inhibition of ADK, a selective ADK inhibitor, ABT702, was administered to Apoe−/− mice following guide wire transluminal injury of the carotid artery. Three weeks following arterial injury, a markedly decreased arterial neointima was observed in Apoe−/− mice treated with ABT702 as compared to mice treated with vehicle (Figure 6I and J). No marked differences in medial wall area were observed between vehicle and ABT702-treated groups (Figure 6K).
3.9 ADK expression associates with DNA hypermethylation in human arterial neointima
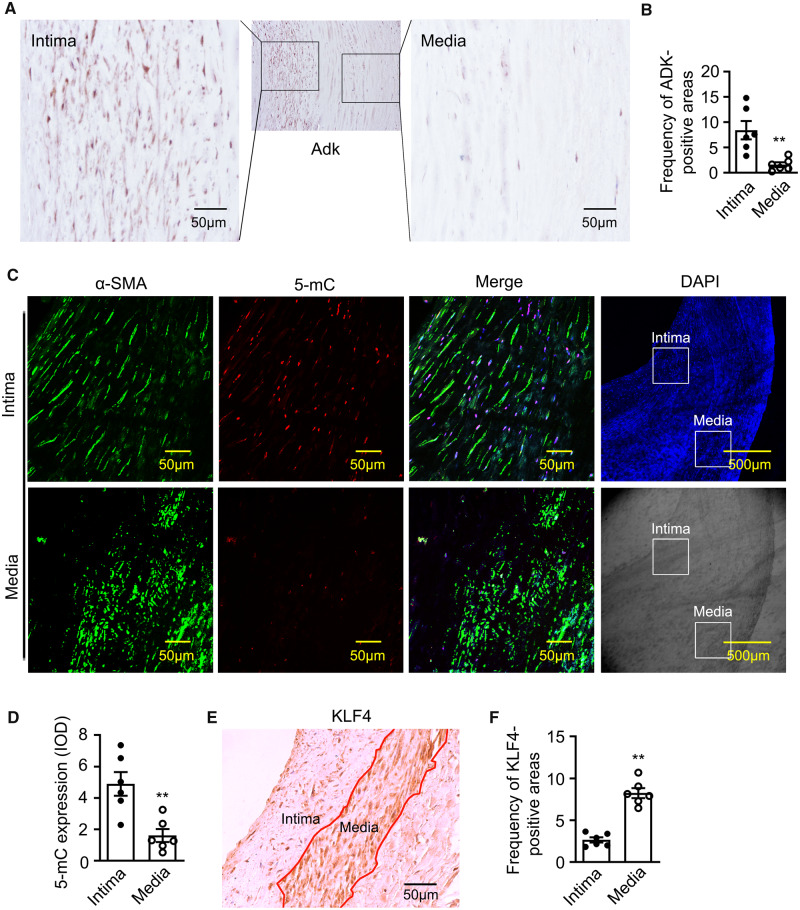
While our data suggest that ADK expression levels can influence DNA methylation and the proliferative capacity of VSMCs in models of vascular disease in mice, whether similar pathways are operational in human blood vessels is not yet known. Therefore, we investigated the expression levels of ADK and the level of 5-mC in human stenotic femoral artery specimens. IHC analysis of ADK in human blood vessels revealed that the levels of ADK were higher in the tunica intima vs. the tunica media (Figure 7A and B). Furthermore, immunofluorescent (IF) staining showed that increased expression of ADK was primarily localized to the nuclei of neointima VSMCs, which were identified by α-SMA staining (Supplementary material online, Figure S4). To explore the relationship between ADK expression and DNA methylation, we measured the level of 5-mC. IF analysis showed significantly higher level of 5-mC immunoreactivity in the tunica intima vs. the tunica media (Figure 7C and D). In contrast, IHC analysis showed that the levels of KLF4 in the tunica intima were lower than those in the tunica media (Figure 7E and F). These data reveal possible associations among ADK level, DNA hypermethylation, and KLF4 level in human arterial neointima and suggest an association between ADK expression level and human proliferative vascular disease.
Figure 7.
ADK expression and DNA methylation in human femoral arterial neointima. (A) Representative IHC staining of ADK on sections of human femoral artery specimens with in-stent restenosis. The left image shows the tunica intima area and the right one shows the tunica media area. ADK-positive cells (brown spots) were shown with DAB staining. (B) Quantification of human stenotic artery specimen in (A) via Image Pro Plus (n = 6 per group). (C and D) Representative IF staining (C) and quantification (D) of 5-mC (red) in the tunica intima area and tunica media area of human stenotic femoral artery (n = 6 per group). (E and F) Representative IHC staining (E) and quantification (F) of KLF4 in the tunica intima area and tunica media area of human stenotic femoral artery (n = 6 per group). For all bar graphs, data are expressed as the means ± SEM, **P < 0.01 (unpaired, two-tailed Student’s t-test).
4. Discussion
To the best of our knowledge, this study is the first to report that ADK overexpression in VSMCs promotes the hypermethylation of DNA and the acquisition of a proliferative phenotype that contributes to arterial neointima formation. Elevated levels of intracellular adenosine achieved either by ADK KD or inhibition promotes DNA hypomethylation that suppresses VSMC proliferation and reduces arterial neointima formation. These findings provide new insights into a previously unrecognized effect of ADK and intracellular adenosine on VSMC proliferation via modification of epigenetic pathways (Supplementary material online, Figure S5A and B).
4.1 SAM-dependent transmethylation pathway promotes a proliferative phenotype in VSMCs and arterial neointima formation
ADK favours SAM-dependent transmethylation reactions by keeping the levels of intracellular adenosine low.13,14 In addition, our recent published study showed that ADK associates with and coordinates with SAHH to remove adenosine that is generated by the SAHH-dependent hydrolysis of SAH, which then facilitates the SAM-dependent transmethylation pathway.23 In this study, deletion of ADK promoted the elevation of intracellular adenosine as well as SAH. SAH is a powerful inhibitor of SAM-dependent transmethylation reactions,24 which accounts for the ability of ADK deletion to inhibit the transmethylation pathway. Functionally, the outcome of a loss in ADK expression in VSMCs is to reduce VSMC proliferation and neointima formation. Previous studies have demonstrated that inhibition of SAM-dependent transmethylation pathways by the SAHH inhibitor, 3-deazaadenosine, suppresses VSMC proliferation and neointima formation.25 Together, these findings indicate that the SAM-dependent transmethylation pathway robustly regulates the proliferative phenotype of VSMCs and influences the extent of arterial neointima formation.
4.2 DNA hypermethylation promotes the VSMC proliferative phenotype and arterial neointima formation
Epigenetic regulation, including DNA methylation, is critically involved in the modulation of VSMC function.7,26,27 Several reports have shown that promoter hypermethylation of individual anti-proliferative genes occurs in diseased arteries and contributes to VSMC hyperplasia.28,29 A few recent reports have indicated that inhibition of DNA methylation has a net anti-proliferative effect on SMCs. The DNMT inhibitor, 5-Aza, has been shown to suppress PDGF-induced proliferation of rat airway SMCs and mouse VSMCs.30,31 In addition, overexpression of the methylcytosine dioxygenase TET2, which oxidizes DNA 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), leading to DNA demethylation,32,33 promotes the expression of anti-proliferative genes in VSMCs and reduces cell proliferation.33 In agreement with these studies, we observed a marked increase in DNMT activity and 5-mC levels in PDGF-treated VSMCs as well as in the size of the arterial neointima. We also observed that inhibition of DNA methylation with the DNMT inhibitor 5-Aza suppressed VSMC proliferation and reduced neointima formation. Therefore, our data suggest that DNA hypermethylation is functionally linked to the proliferative phenotype of VSMCs and neointima formation.
4.3 Inhibition of DNA hypermethylation, through elevation of intracellular adenosine levels, contributes to the suppression of VSMC proliferation and arterial neointima formation in ADK KD VSMCs
As previously shown in neurons and astrocytes,14 ADK levels positively correlate with DNA hypermethylation in VSMCs. As the major adenosine metabolizing enzyme, the loss of ADK in VSMCs led to an increased level of intracellular adenosine that then biochemically represses SAM-dependent DNA transmethylation reactions through product inhibition (Supplementary material online, Figure S1A). Using an ELISA-based assay as well as a genome-wide Infinium methylation assay, we found that ADK KD decreased the global DNA methylation status in VSMCs. Furthermore, we found that the actions of ADK KD to inhibit VSMC proliferation and reduce the neointima formation mimicked those of the DNA methylation inhibitor 5-Aza. This data suggests that the compromised proliferation of VSMCs and the constrained arterial neointima formation secondary to ADK deficiency can, at least partially, be attributed to adenosine-dependent DNA hypomethylation.
4.4 KLF4 is one of the key mediators for intracellular adenosine to suppress VSMC proliferation
Previous studies have suggested that KLF4 functions not only as a repressor of VSMC differentiation but also as a VSMC growth repressor.34 Owen et al.35 found that KLF4 inhibits VSMC differentiation through transcriptional repression of the expression of VSMC marker genes. Many studies have shown that increased expression of KLF4 inhibits the proliferation of VSMCs. In a carotid artery ligation model, Yoshida et al.18 reported that although a conditional deletion of KLF4 delays the down-regulation of smooth muscle cell differentiation markers, it increases VSMC proliferation and enhances arterial neointima formation, indicating that KLF4 is a negative regulator of VSMC proliferation in vivo. In our study, ADK KD VSMCs exhibited not only a low rate of proliferation but also low levels of the VSMC markers α-actin and SM22α compared with control VSMCs (Figure 3E and F). The phenotypes of ADK KD VSMCs were similar to those of KLF4-overexpressing VSMCs, suggesting a functional relationship between ADK KD and KLF4 overexpression. Among the anti-proliferative genes for VSMCs that exhibited reduced promoter methylation in ADK-deficient cells, the KLF4 level was the most robustly up-regulated. In addition, KLF4 was also the most up-regulated anti-proliferative gene in cells treated with the DNA methylation inhibitor 5-Aza, indicating that in VSMCs the KLF4 promoter locus is a methylation/demethylation hotspot. The significantly reduced level of methylation on the KLF4 promoter in ADK KD VSMCs was confirmed by bisulfite sequencing. We also found that short-term PDGF treatment increased, but long-term PDGF treatment decreased, the transcription of KLF4. Bisulfite sequencing data suggest that the reduced transcription of KLF4 results from DNA hypermethylation triggered by prolonged PDGF treatment. These data indicate that down-regulation of KLF4 by ADK-mediated potentiation of DNA hypermethylation contributes to the PDGF-induced VSMC proliferative phenotype. In addition, KD of KLF4 in ADK KD VSMCs partially compromises the anti-proliferative effect of ADK KD and provides evidence to support the links between KFL4 promoter hypomethylation, increased KLF4 expression, and compromised proliferation of ADK KD VSMCs.
4.5 ADK-deficiency-induced suppression of VSMC proliferation and arterial neointima formation is mediated by cooperative interaction between extracellular adenosine-mediated adenosine receptor activation and intracellular adenosine-mediated DNA hypomethylation
We have shown that the hypomethylation of DNA triggered by ADK KD/inhibition in VSMCs is independent of adenosine receptor A2AR and A2BR, which is consistent with previous reports in other cell types.14,36 However, the current study cannot rule out the possibility that ADK deletion has additional distinct effects on adenosine receptor expression on VSMCs and that autocrine activation of adenosine receptors, especially A2BR, may inhibit VSMC proliferation. Studies in vitro have demonstrated that A2BR activation mediates the anti-proliferative effects of exogenous or endogenous (VSMC-derived) adenosine in VSMCs.17,37 Using a femoral artery ligation model of vascular disease, Yang et al.38 reported that A2BR deficiency exacerbated neointima formation by promoting VSMC proliferation, which suggests that A2BR activation suppresses VSMC proliferation in vivo. In our study, we found that the anti-proliferative effect of ADK deletion was compromised by KLF4 deficiency, but not by genetic or pharmacological inhibition of A2BR, implying that KLF4 up-regulation due to DNA hypomethylation, but not A2BR activation, is the dominant mediator of the anti-proliferative effect of ADK deletion. However, considering the increased extracellular adenosine levels (Figure 1J) and increased intracellular cAMP levels (Supplementary material online, Figure S3I) observed post-ADK deletion, the involvement of autocrine activation of A2BR in the anti-proliferative effect of ADK deletion remains a possibility. In addition, paracrine/autocrine adenosine signalling would be much more anticipated in in vivo studies. The elevated extracellular adenosine induced by VSMC ADK deficiency may exert paracrine and autocrine anti-proliferative functions on most vascular cell types.
4.6 ADK could be an important target for therapeutic augmentation of intracellular adenosine and inhibition of aberrant DNA methylation to treat proliferative and remodelling vascular diseases
In the present study, we demonstrate that pharmacological inhibition of the aberrant DNA hypermethylation by targeting ADK prevents neointima formation but does not increase VSMC apoptosis and necrosis, indicating that ADK is a potential therapeutic target. Increased expression of ADK in the human stenotic femoral artery suggests that ADK may also contribute to pathological remodelling and arterial neointima development in humans. Despite great efforts aimed at targeting epigenetic pathways for the treatment of vascular diseases, no related agents have been tested in clinical trials for the treatment of vascular diseases thus far. Among many epigenetic molecules characterized to regulate DNA methylation, none of them are targetable for treating vascular diseases. With enzymatic activity that can be inhibited by small molecules, the targetable properties of ADK have already received much attention in drug development for the treatment of seizure and pain.39,40 Thus, targeting ADK for treatment of arterial neointima is warranted. The current medicines used in medicated stents for treating patients with coronary artery disease, such as rapamycin and paclitaxel, reduce not only neointima formation but also endothelial cell repopulation, leading to clinical concerns over delayed arterial healing, poor re-endothelialization, and late stent thrombosis.41,42 In contrast, our recent studies show targeting endothelial ADK increases the rate of endothelial cell repopulation and improves endothelial cell function.23,43–45 Overall, these findings indicate that targeting ADK to therapeutically augment intracellular adenosine may represent an attractive and safe therapeutic strategy for the treatment of proliferative and remodelling vascular diseases.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
Y.W., Y.X., and Y.H. designed research. Y.W., Y.X., K.C., S.Y., Y.Z., X.Z., Z.L., and Q.Y. performed research. Y.W., Y.X., S.Y., Y.P., and X.W. analysed data. Y.X., D.B., Y.S., X.J., V.S.P., C.W. D.F., N.L.W., and Y.H. wrote the manuscript.
Conflict of interest: none declared.
Funding
This work was supported by the Natural Science Foundation of China (81870363, 81870217, 81700395, and 81400826), National Institutes of Health (HL095556, R01DK095828, and R01DK095862), American Heart Association (16GRNT30510010 and 15POST22810024), National Guangdong Natural Science Foundation (2014A030312004), and Shenzhen Science and Technology Innovation Committee (JSGG20140717102922014, JCYJ20140903101709818, KQCX2015032709315529, 20160517084712652, and 20160503001803075).
Supplementary Material
Translational perspective
Abnormal proliferation of vascular smooth muscle cell (VSMC) is key to abundant occlusive vascular diseases in humans, such as atherosclerosis and intimal hyperplasia associated with restenosis. Adenosine has been shown to combat abnormal smooth muscle proliferation. Here, we demonstrate that increased catabolism of adenosine by adenosine kinase (ADK) promotes abnormal VSMC proliferation. The pathological ADK overexpression in both mice and humans with vascular disease promotes VSMC proliferation via inducing aberrant DNA hypermethylation and KLF4 down-regulation. Our study suggests that pharmacological augmentation of endogenous adenosine by targeting ADK represents a promising therapeutic strategy for occlusive vascular diseases.
References
- 1. Goldberg ID, Stemerman MB, Schnipper LE, Ransil BJ, Crooks GW, Fuhro RL.. Vascular smooth muscle cell kinetics: a new assay for studying patterns of cellular proliferation in vivo. Science 1979;205:920–922. [DOI] [PubMed] [Google Scholar]
- 2. Clowes AW, Reidy MA, Clowes MM.. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest 1983;49:327–333. [PubMed] [Google Scholar]
- 3. Herring BP, Hoggatt AM, Burlak C, Offermanns S.. Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc Cell 2014;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clowes AW, Reidy MA, Clowes MM.. Mechanisms of stenosis after arterial injury. Lab Invest 1983;49:208–215. [PubMed] [Google Scholar]
- 5. Chaabane C, Otsuka F, Virmani R, Bochaton-Piallat ML.. Biological responses in stented arteries. Cardiovasc Res 2013;99:353–363. [DOI] [PubMed] [Google Scholar]
- 6. Alexander MR, Owens GK.. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74:13–40. [DOI] [PubMed] [Google Scholar]
- 7. Liu R, Leslie KL, Martin KA.. Epigenetic regulation of smooth muscle cell plasticity. Biochim Biophys Acta 2015;1849:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK.. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest 2005;116:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR.. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H.. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest 2014;124:3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao Q, Wang X, Jia L, Mondal AK, Diallo A, Hawkins GA, Das SK, Parks JS, Yu L, Shi H, Shi H, Xue B.. Inhibiting DNA Methylation by 5-Aza-2’-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology 2014;155:4925–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boison D, Scheurer L, Zumsteg V, Rulicke T, Litynski P, Fowler B, Brandner S, Mohler H.. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA 2002;99:6985–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boison D. Adenosine kinase: exploitation for therapeutic gain. Pharmacol Rev 2013;65:906–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D.. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Invest 2013;123:3552–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW.. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest 1992;89:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dubey RK, Gillespie DG, Mi Z, Suzuki F, Jackson EK.. Smooth muscle cell-derived adenosine inhibits cell growth. Hypertension 1996;27:766–773. [DOI] [PubMed] [Google Scholar]
- 17. Dubey RK, Gillespie DG, Shue H, Jackson EK.. A(2B) receptors mediate antimitogenesis in vascular smooth muscle cells. Hypertension 2000;35:267–272. [DOI] [PubMed] [Google Scholar]
- 18. Yoshida T, Kaestner KH, Owens GK.. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 2008;102:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, Su M, Han Y, Shi HJ, Wen JK.. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep 2011;12:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Y, Zhao B, Zhang Y, Tang Z, Shen Q, Zhang Y, Zhang W, Du J, Chien S, Wang N.. Kruppel-like factor 4 is induced by rapamycin and mediates the anti-proliferative effect of rapamycin in rat carotid arteries after balloon injury. Br J Pharmacol 2012;165:2378–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou AX, Wang X, Lin CS, Han J, Yong J, Nadolski MJ, Boren J, Kaufman RJ, Tabas I.. C/EBP-homologous protein (CHOP) in vascular smooth muscle cells regulates their proliferation in aortic explants and atherosclerotic lesions. Circ Res 2015;116:1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng WL, She ZG, Qin JJ, Guo JH, Gong FH, Zhang P, Fang C, Tian S, Zhu XY, Gong J, Wang ZH, Huang Z, Li H.. Interferon regulatory factor 4 inhibits neointima formation by engaging Kruppel-like factor 4 signaling. Circulation 2017;136:1412–1433. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Wang Y, Yan S, Yang Q, Zhou Y, Zeng X, Liu Z, An X, Toque HA, Dong Z, Jiang X, Fulton DJ, Weintraub NL, Li Q, Bagi Z, Hong M, Boison D, Wu C, Huo Y.. Regulation of endothelial intracellular adenosine via adenosine kinase epigenetically modulates vascular inflammation. Nat Commun 2017;8:943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang J, Zheng YG.. SAM/SAH analogs as versatile tools for SAM-dependent methyltransferases. ACS Chem Biol 2016;11:583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sedding DG, TröBs M, Reich F, Walker G, Fink L, Haberbosch W, Rau W, Tillmanns H, Preissner KT, Bohle RM, Langheinrich AC.. 3-Deazaadenosine prevents smooth muscle cell proliferation and neointima formation by interfering with Ras signaling. Circ Res 2009;104:1192–1200. [DOI] [PubMed] [Google Scholar]
- 26. Hiltunen MO, Yla-Herttuala S.. DNA methylation, smooth muscle cells, and atherogenesis. Arterioscler Thromb Vasc Biol 2003;23:1750–1753. [DOI] [PubMed] [Google Scholar]
- 27. Elia L, Kunderfranco P, Carullo P, Vacchiano M, Farina FM, Hall IF, Mantero S, Panico C, Papait R, Condorelli G, Quintavalle M.. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J Clin Invest 2018;128:2473–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ying AK, Hassanain HH, Roos CM, Smiraglia DJ, Issa JJ, Michler RE, Caligiuri M, Plass C, Goldschmidt-Clermont PJ.. Methylation of the estrogen receptor-alpha gene promoter is selectively increased in proliferating human aortic smooth muscle cells. Cardiovasc Res 2000;46:172–179. [DOI] [PubMed] [Google Scholar]
- 29. Wang YS, Chou WW, Chen KC, Cheng HY, Lin RT, Juo SH.. MicroRNA-152 mediates DNMT1-regulated DNA methylation in the estrogen receptor alpha gene. PLoS One 2012;7:e30635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ning Y, Huang H, Dong Y, Sun Q, Zhang W, Xu W, Li Q.. 5-Aza-2’-deoxycytidine inhibited PDGF-induced rat airway smooth muscle cell phenotypic switching. Arch Toxicol 2013;87:871–881. [DOI] [PubMed] [Google Scholar]
- 31. Zhuang J, Luan P, Li H, Wang K, Zhang P, Xu Y, Peng W.. The Yin-Yang dynamics of DNA methylation is the key regulator for smooth muscle cell phenotype switch and vascular remodeling. Arterioscler Thromb Vasc Biol 2017;37:84–97. [DOI] [PubMed] [Google Scholar]
- 32. Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y.. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011;333:1300–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA.. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation 2013;128:2047–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zheng B, Han M, Wen JK.. Role of Kruppel-like factor 4 in phenotypic switching and proliferation of vascular smooth muscle cells. IUBMB Life 2010;62:132–139. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK.. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem 2005;280:9719–9727. [DOI] [PubMed] [Google Scholar]
- 36. Huang A, Wu H, Iriyama T, Zhang Y, Sun K, Song A, Liu H, Peng Z, Tang L, Lee M, Huang Y, Ni X, Kellems RE, Xia Y.. Elevated adenosine induces placental DNA hypomethylation independent of A2B receptor signaling in preeclampsia. Hypertension 2017;70:209–218. [DOI] [PubMed] [Google Scholar]
- 37. Dubey RK, Fingerle J, Gillespie DG, Mi Z, Rosselli M, Imthurn B, Jackson EK.. Adenosine attenuates human coronary artery smooth muscle cell proliferation by inhibiting multiple signaling pathways that converge on cyclin D. Hypertension 2015;66:1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K.. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci USA 2008;105:792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kowaluk EA, Bhagwat SS, Jarvis MF.. Adenosine kinase inhibitors. Curr Pharm Des 1998;4:403–416. [PubMed] [Google Scholar]
- 40. McGaraughty S, Cowart M, Jarvis MF, Berman RF.. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem 2005;5:43–58. [DOI] [PubMed] [Google Scholar]
- 41. Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, Serruys P.. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol 2004;44:733–739. [DOI] [PubMed] [Google Scholar]
- 42. Douglas G, Van Kampen E, Hale AB, McNeill E, Patel J, Crabtree MJ, Ali Z, Hoerr RA, Alp NJ, Channon KM.. Endothelial cell repopulation after stenting determines in-stent neointima formation: effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur Heart J 2013;34:3378–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gu JW, Ito BR, Sartin A, Frascogna N, Moore M, Adair TH.. Inhibition of adenosine kinase induces expression of VEGF mRNA and protein in myocardial myoblasts. Am J Physiol Heart Circ Physiol 2000;279:H2116–H2123. [DOI] [PubMed] [Google Scholar]
- 44. Xu Y, Wang Y, Yan S, Zhou Y, Yang Q, Pan Y, Zeng X, An X, Liu Z, Wang L, Xu J, Cao Y, Fulton DJ, Weintraub NL, Bagi Z, Hoda MN, Wang X, Li Q, Hong M, Jiang X, Boison D, Weber C, Wu C, Huo Y.. Intracellular adenosine regulates epigenetic programming in endothelial cells to promote angiogenesis. EMBO Mol Med 2017;9:1263–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang M, Zeng X, Yang Q, Xu J, Liu Z, Zhou Y, Cao Y, Zhang X, An X, Xu Y, Huang L, Han Z, Wang T, Wu C, Fulton DJ, Weintraub NL, Hong M, Huo Y.. Ablation of myeloid ADK (adenosine kinase) epigenetically suppresses atherosclerosis in ApoE-/- (apolipoprotein E deficient) mice. Arterioscler Thromb Vasc Biol 2018;38:2780–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.