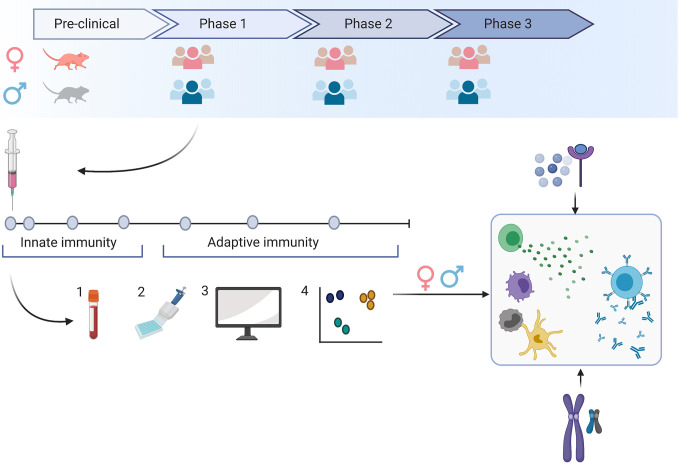
Figure 1.
Sex-specific immune responses can lead to vaccine responses that differ in safety, immunogenicity or efficacy. To evaluate the impact of sex on the specific vaccine candidate, we need to include both sexes as early as during the preclinical development stage and aim for a balanced sex ratio in clinical phase 1-3 trials. A detailed snapshot of immune responses to the vaccine can be achieved by frequent blood sampling following vaccination (1) and the application of various technologies (2). Here, we can evaluate responses to the vaccine on the transcriptome, epigenome and proteome level. Using bioinformatic tools (3), we may gain a comprehensive insight into multiple levels of immune responses that may be different in men and women (4). By comprehensively studying innate and adaptive immune responses in men and women, we may better understand how genetic or hormonal differences affect the number and functionality of immune cells.