Abstract
Kinetic and physicochemical properties of Moringa oleifera peroxidase purified using a novel and cost efficient protocol was investigated with a view to providing information on its possible biotechnological potentials.
Moringa oleifera peroxidase was purified to homogeneity in two steps, involving ATPS and size exclusion chromatography on Sephadex G-100 with a yield of 84.12 %. In-gel activity staining revealed the presence of one isoform of peroxidase. The purified peroxidase is monomeric with native and subunits molecular weight of 38.9 and 43.5 kDa respectively. Kinetic parameters - Vmax, Km(app)o-dianisidine, Km(app)H2O2 of the purified enzyme were 2.5 units/mg protein, 0.020 ± 0.04 mM and 1.37 ± 0.18 mM respectively. Its optimum pH and temperature were 5 and 30 °C respectively. The purified enzyme cross-linked BSA into an insoluble matrix with the aid of caffeic acid.
The study concluded that the purification scheme adopted is rapid and efficient, the purified enzyme exhibited some physiochemical properties that make it suitable for various biotechnological applications.
Keywords: Moringa oleifera, Peroxidase, Aqueous two-phase system (ATPS), Reporter enzyme, Cross-linked protein network
Moringa oleifera; Peroxidase; Aqueous two-phase system (ATPS); Reporter enzyme; Cross-linked protein network
1. Introduction
Peroxidases (EC 1.11.1.x) are a group of enzymes that contain heme, and they oxidize varieties of xenobiotics by using hydrogen peroxide (Saunders, 1973). Peroxidase is an anti-oxidative enzyme that is widely distributed in microbe, plant, and animal tissue. They represent a large group of heme-containing enzymes family (Van Huystee and Cairns, 1982). This oxido-reductase catalyzes a reaction in which hydrogen peroxide acts as the acceptor and another compound acts as the donor of hydrogen atoms (Rodrigo et al., 1996). In the presence of peroxide, peroxidase from plant tissues can oxidize a wide range of phenolic compounds, such as guaiacol, catechol, pyrogallol, chlorogenic acid, and catechin (Onsa et al., 2004). Peroxidase has a diverse function in the plant. They are involved in the lignifications of cell walls (Adewale and Adekunle, 2018), plant differentiation and growth, plant defense from a pathogenic microorganism, and wound healing (Shigeto and Tsutsumi, 2016). Other functions of peroxidase include their involvement in the biotransformation of drugs, chemicals, and polymers (Sakai et al., 2014). Also, their ability to oxidize phenolic has increased its use for various industrial applications, of which the most important ones include decolorization of waste (Lai and Lin, 2005; Dalal and Gupta, 2007; Jadhav et al., 2009), synthesis of various aromatic chemicals, removal of peroxides from foodstuff, industrial wastes and crosslinking of macromolecules (proteins) (Saitou et al. 1991; Kim and Yoo, 1996) and in the biological field, as diagnostic kits for enzyme immunoassays and as an important component of ELISA system (Leon et al., 2002; Deepa and Arumughan, 2002).
Horseradish (Armoracia rusticana) roots are the major sources of commercially available peroxidase. Due to their biochemical diversity, they are used as a traditional source of peroxidase for commercial production. They exist in multiple isoforms which makes it a convenient toolbox of plant peroxidases from which an isoenzyme that meets the requirements of an application can be chosen (Adewale and Adekunle, 2018). The combination of various features in the horseradish toolbox with the aid of recombinant technology allows for improved biocatalysis and novel synthesis in the use of peroxidase, however, this is still at the infancy. Thus, numerous studies have been carried out in a search for an alternative source of peroxidase with higher stability, availability, degree of purification, substrate specificity and novel properties.
Moringa oleifera (English name: drumstick tree) is a medium-sized tree with small-sized leaves native to Bangladesh, China, Nepal Pakistan, India, but also cultivated in tropical America, tropical Africa, Malaysia, and the Philippines and its widely known for its medicinal value (Popoola and Obembe, 2013). The flowers and fruits of the Moringa oleifera plant are rich in nutrients. The leaves of the Moringa oleifera plant are rich in vitamins, carotenoids, polyphenols, phenolic acids, flavonoids, alkaloids, glucosinolates, isothiocyanates, tannins, and saponins (Leone et al., 2015). All parts of the Moringa oleifera plant; the root, bark, gum, leaf, pod, and seeds, have been reported to have various biological activities. Generally Moringa oleifera has diverse medicinal and biomedical applications. The plant extract has been reported in the synthesis of nanoparticle which posses better cytotoxicity and antibacterial activity (Ezhilarasi et al., 2016), and have been used traditionally in the purification of water and treatment of various diseases from malaria and typhoid fever to hypertension and diabetes (Sivasankari et al., 2014).
Previous work by Khatun et al. (2012) had shown the presence of peroxidase in Moringa oleifera leaves with desirable properties. This study, however focuses on a rapid purification scheme for the enzyme preparations and its possible biotechnological potential.
2. Materials and method
2.1. Materials
Sephadex G-100 and Sephacryl S-300 were obtained from Pharmacia Fine Chemicals, Uppsala, Sweden. The molecular weight standard for SDS-PAGE was obtained from Thermo Scientific, Lithuania. Caffeic acid, bovine serum albumin, ammonium sulphate, and polyethylene glycol (PEG 6000) were obtained from Sigma Chemical, St Louis, USA. All other reagents were of analytical grade and were obtained from reputable chemical suppliers. Moringa oleifera leaves were harvested from the Moringa oleifera tree within Obafemi Awolowo University Campus Ile-Ife.
2.2. Methods
2.2.1. Enzyme extraction and measurement of enzyme activity
Enzyme extraction from young and matured leaves of Moringa oleifera was carried out in 10 mM phosphate buffer pH 6.0 containing 10% glycerol at 4 °C for 1 min in a warring blender. The homogenate was centrifuged at 10,000 x g for 30 min at 4 ᵒC to obtain a cell debris-free supernatant which was stored as crude enzyme homogenate. Peroxidase activities were routinely determined according to the method of Kay et al. (1967) as described by Adewale and Adekunle (2018). Briefly, the reaction mixture contained in final concentration, hydrogen peroxide (1 mM), o-dianisidine (0.25 mM), 0.1 M sodium phosphate buffer pH 6.0, and enzyme aliquot that gave a change in absorbance of 0.02–0.07 per min at 460 nm as a result of the oxidation of o-dianisidine in the presence of hydrogen peroxide. One unit of enzyme activity is defined as the amount of enzyme that oxidizes 1 μmol o-dianisidine/min (ε460 = 11.3 mM−1 cm−1). The leave homogenate with the highest peroxidase activity was adopted for further studies.
2.2.2. Protein concentration determination
Protein concentration was routinely measured according to the method of Bradford (1976) using bovine serum albumin as the standard protein. The protein standard curve was determined by pipetting from 0 – 1.0 ml of BSA (10 μg/ml) into labeled test tubes equivalent to 0–10 μg BSA concentration. Afterwards, the test-tube was made up to 1.8 ml with distilled water. Then, 0.2 ml of Bradford reagent was added to each of the test tubes, which was then mixed and incubated briefly at room temperature. The absorbance of each test tube was read against a blank which contained all other components of the mixture except BSA at 595 nm. Absorbance was then plotted against the corresponding amount (mg) of standard protein to construct the standard curve. Protein concentration in the supernatant was determined by extrapolation from the standard protein curve.
2.2.3. In-gel activity staining of peroxidase
In-gel activity staining for the determination of the presence of peroxidase in the crude and the purified sample was carried out on a native polyacrylamide gel electrophoresis (native PAGE) on 10% separating gel and a 4% stacking gel using the Tris-glycine buffer system (25 mM Tris-base and 192 mM Glycine) at pH 8.3 as described by Laemmli (1970). The crude and purified peroxidase samples were prepared by pipetting 30 μl each of crude and purified peroxidase and 30 μl of sample buffer (0.12 M Tris-base pH 6.8 containing 10% glycerol and 0.02% bromophenol blue). Aliquots of the resulting mixtures were loaded separately on a slab gel. Electrophoresis was carried out at room temperature at a constant voltage of 100 mV for the stacking gel and 150 mV for the separating gel. After electrophoresis, gels were immersed into a solution containing a final concentration of 30 mM H2O2, 15 mM o-dianisidine, and 0.1 M sodium phosphate buffer pH 6.5 for 10 min for the development of chromophores. Thereafter, the gel was removed, washed in distilled water, and viewed for band formation.
2.2.4. Purification of peroxidase
The purification of peroxidase from Moringa oleifera was carried out using a combination of aqueous two-phase partitioning system and gel filtration chromatography.
Purification by aqueous two-phase partitioning system (ATPS) was carried out according to the method of Srinivas et al. (2002) with slight modification. Polyethylene glycol (PEG 6000, 35% w/v), ammonium sulphate (7.5% w/v) and sodium chloride (2% w/v) were dissolved in the crude extract on ice. The mixture was stirred continuously to achieve a completely homogenous mixture which was then incubated on ice for phase separation. The top and bottom phases were collected and were assayed for peroxidase activity and protein concentration. The salt-rich bottom phase which had higher peroxidase activity was dialyzed against four changes of buffer (10 mM phosphate buffer pH 6.0 containing 10% glycerol) at 4 °C for 6 h in a cold box to remove salts.
The partially purified sample obtained from dialysis was further purified on Sephadex G-100 (1 × 50 cm) gel filtration column which was previously equilibrated with 10 mM sodium phosphate buffer pH 6.5 containing 10% glycerol.
2.2.5. Determination of native and subunit molecular weight
The native molecular weight of the purified peroxidase was carried out on calibrated Sephadex G-100 and the subunits molecular weight of the purified peroxidase was determined by SDS-polyacrylamide gel electrophoresis on a 12 % (w/v) polyacrylamide running gel and a 4 % (w/v) polyacrylamide stacking gel according to the method of Laemmli (1970) along with Thermo Scientific Molecular weight marker.
2.2.6. Determination of kinetic parameters
The effect of hydrogen peroxide and o-dianisidine on the purified peroxidase from Moringa oleifera was determined by assaying for peroxidase activity at varying concentrations of hydrogen peroxide between 0 mM–5 mM at a constant concentration of o-dianisidine (0.25 mM) and also by varying the concentrations of o-dianisidine between 0 mM–0.25 mM at a constant concentration of hydrogen peroxide (1 mM) in 50 mM phosphate buffer. Apparent kinetic parameters Km and Vmax for both substrates were interpolated using non-linear regression software (GraphPad Prism 5).
2.2.7. Effect of temperature on peroxidase activity
The effect of temperature on peroxidase activity was studied by incubating reaction mixtures containing 10 mM phosphate buffer pH 6.0, 0.25 mM o-dianisidine, and 1 mM H2O2 at the temperature range of 20 °C–50 °C in a water bath for 10 min. The enzyme was introduced into the medium, stirred, and then placed in the spectrophotometer to take the absorbance. A graph of activity against the respective values of temperature was plotted and the optimum temperature was interpolated.
2.2.8. Effect of pH on peroxidase activity
The effect of the pH on the enzyme activity was performed according to the methods of Adewale and Adekunle (2018). The activity was determined in the pH range of 3.0–10.0 at 30 °C. The following buffer systems at the indicated pH ranges were used: 50 mM acetate buffer pH 3.0–5.0, 50 mM MES buffer pH 5.5–6.5, 50 mM phosphate buffer pH 7.0–8.0, and 50 mM borate buffer pH 8.5–10.0. The peroxidase activity was assayed as described by Kay et al. (1967) with the assay buffer being replaced by each of these buffers.
2.2.9. Synthesis of cross-linked protein networks
The potential of the purified peroxidase in the synthesis of cross-linked protein network was carried out using BSA as the protein at varying concentrations (1.0 mg/ml, 10 mg/ml) and in the presence of caffeic acid (0.5 mM, 1.0 mM, 2.0 mM) and a fixed concentration of H2O2 as the substrate. The reaction mixture of 0.5 ml contained BSA, caffeic acid, H2O2, 50 mM phosphate buffer, and an aliquot of purified peroxidase. Control reaction mixtures were prepared in the absence of the purified enzyme and substrate. The mixtures were incubated at 50 °C overnight (18 h). The products were observed with a Zeiss LSM 510 META confocal microscope fitted to a Zeiss Axiovert 200 M.
2.2.10. Cross-linking of peroxidase with BSA
The potentials of Moringa oleifera peroxidase as a reporter enzyme was investigated by coupling the enzyme with BSA. This was carried out according to the method of Ayhan et al. (2012) as described by Adewale and Adekunle (2018). Briefly, this was done by mixing 0.3 mg/ml BSA and 0.2651 mg/ml peroxidase in a 1:1 ratio after which glutaraldehyde (0.25%) was added to the solution. The solution was incubated for 2 h and peroxidase activity was determined as earlier described. The ability of the peroxidase to conjugate BSA was monitored on a calibrated Sephacryl S-300 column.
2.2.11. Statistic
All experiments are carried out at least in triplicate unless otherwise stated. Data were expresses as mean ± standard deviation. Statistical analysis was perform using Graph pad prism 5.0 software.
3. Results
3.1. Expression of peroxidase in young and mature leaves of Moringa oleifera
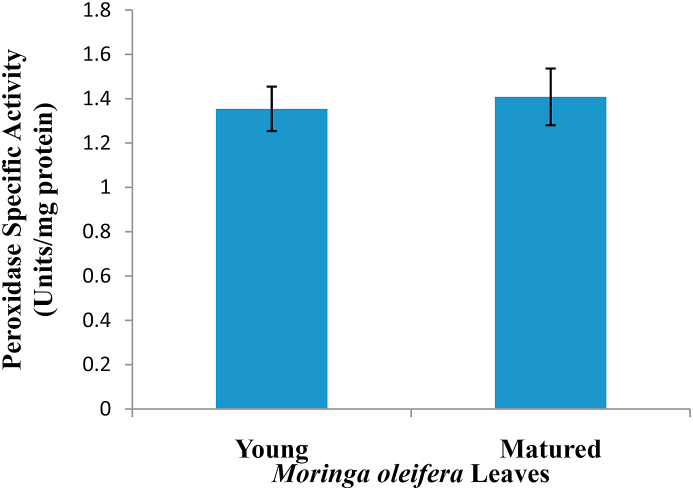
Peroxidase is higher in the leaves of matured leaves of Moringa oleifera (1.4082 units/mg protein) when compared to young leaves (1.3541 units/mg protein). However, the difference is not significant(Figure 1).
Figure 1.
Summary of specific activity of peroxidase from Moringa oleifera leaves.
3.2. In-gel Activity Staining of Peroxidase
In-gel activity staining reveals the presence of only one isoform of peroxidase in the matured leave extract of Moringa oleifera leaves (Figure 2).
Figure 2.

Activity Staining of Peroxidase from Moringa oleifera Leaves. After electrophoresis, gels were immersed into a solution containing a final concentration of 30 mM H2O2, 15 mM o-dianisidine, and 0.1 M sodium phosphate buffer pH 6.5 for the development of chromophores. Where: lane A and B represent the represent crude and purified peroxidase respectively.
3.3. Enzyme purification
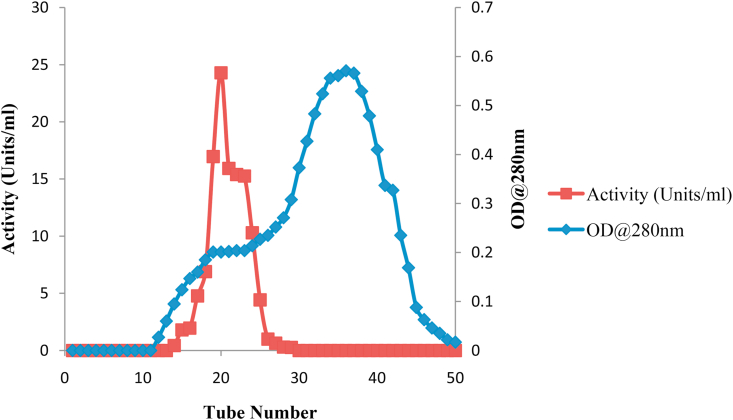
Purification of peroxidase from Moringa oleifera leaves using a combination of ATPS and size exclusion chromatography on Sephadex G-100, resulted in one peroxidase activity peak with final recovery of 84% (Table 1 and Figure 3).
Table 1.
Purification summary of Peroxidase from Moringa oleifera leave.
| Volume (ml) | Activity (μmol/min/ml) | Protein Concentration (mg/ml) | Specific Activity (μmol/min/mg) | Total Activity (μmol/min) | Total Protein (mg) | Purification Fold | Recovery | |
|---|---|---|---|---|---|---|---|---|
| Crude | 86 | 14.0695 ±1.0095 |
3.495 ±0.186 |
4.0256 ±0.125 |
1,209.9 | 300.57 | 1 | 100 |
| Aqueous Two-Phase Partitioning System | 18 | 176.88 ±25.95 |
4.589 ±0.3743 |
38.544 ±0.288 |
3,183.8 | 82.60 | 9.57 | 263.13 |
| Gel Filtration on Sephadex G-100 | 288 | 3.5342 ±0.3550 |
0.250 ±0.05 |
14.1368 ±0.30 |
1,017.8 | 72.00 | 3.51 | 84.12 |
Figure 3.
Elution profile of partially purified peroxidase (Post ATPS) on Sephadex G-100 Column. Purification of Moringa oleifera peroxidase using a combination of ATPS and gel filtration chromatography on Sephadex G-100 resulted in only one peak of activity with percentage recovery of 84 % and purification fold of about 4.
3.4. Subunit molecular weight determination
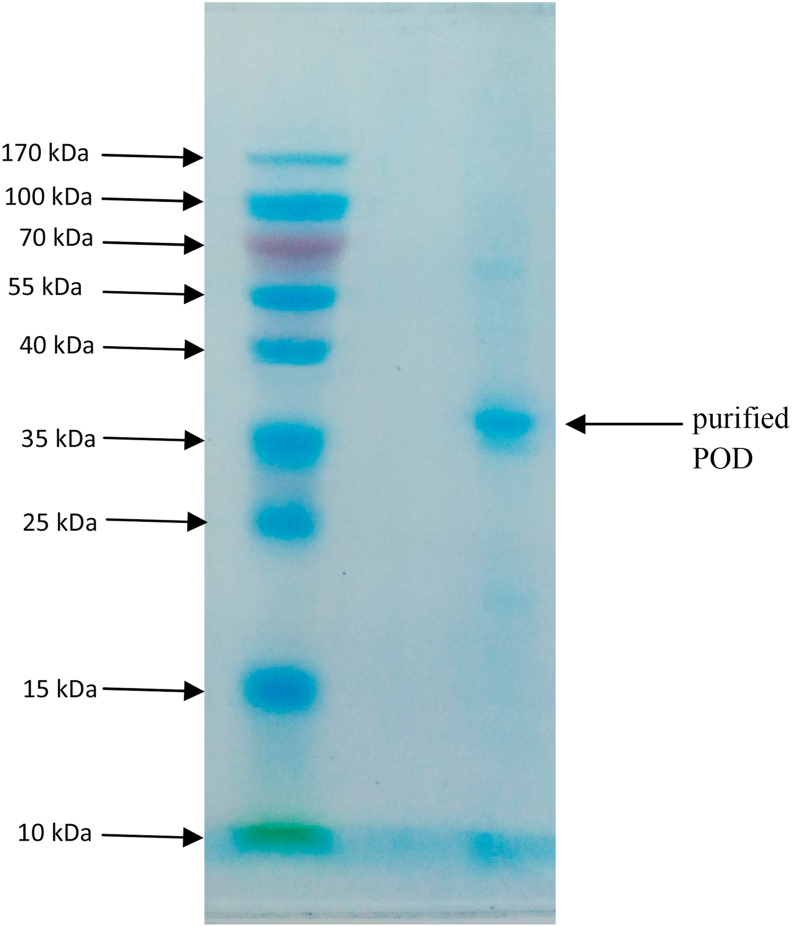
The sub-unit molecular weight of the purified Moringa oleifera peroxidase on a 12% polyacrylamide gel electrophoresis in the presence of SDS gave a single band equivalent to 43.5189 ± 1.6303 kDa(Figure 4).
Figure 4.
Electrophoretogram of purified peroxidase from Moringa oleifera. SDS-PAGE of purified peroxidase from Moringa oleifera leaves was carried out on 12% gel and 4% stacking gel using the SDS–Tris–glycine buffer system (25 mmol. dm−3 Tris base, 192 mmol dm−3 glycine and 0.1% SDS) at pH 8.3. The experiments was carried out at room temperature.
3.5. Native molecular weight determination by gel filtration on Sephadex G-100
The native molecular weight for purified Moringa oleifera peroxidase was 38.98 ± 1.41 kDa.
3.6. Kinetic parameter
The apparent kinetic parameters Km(app) o-dianisidine, Km(app) H2O2 and Vmax for the purified peroxidase were 0.0194 ± 0.041 mM; 1.372 ± 0.1837 mM and 2.4925 ± 0.0902 Units/mg protein respectively.
3.7. Effect of temperature
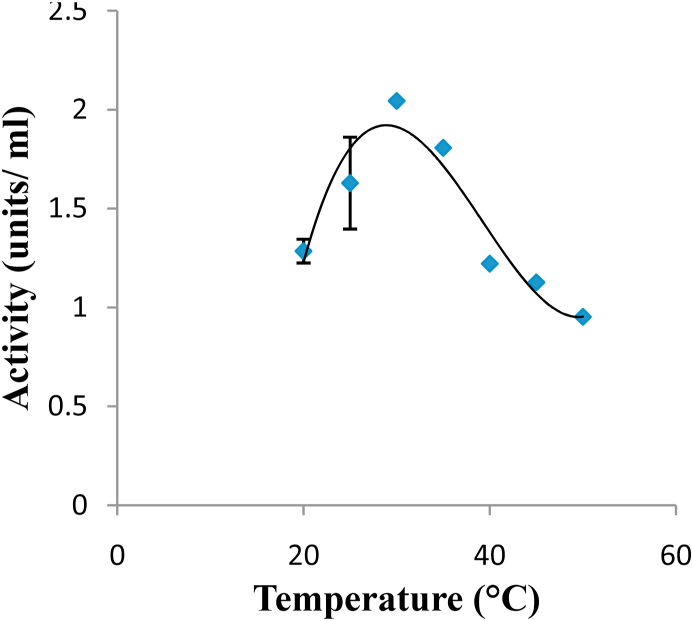
The optimum temperature of the purified peroxidase from Moringa oleifera was 30 ᵒC (Figure 5).
Figure 5.
Effect of temperature on the activity of peroxidase from Moringa oleifera. The optimum temperature of the purified enzyme was 30°C.
3.8. Effect of pH
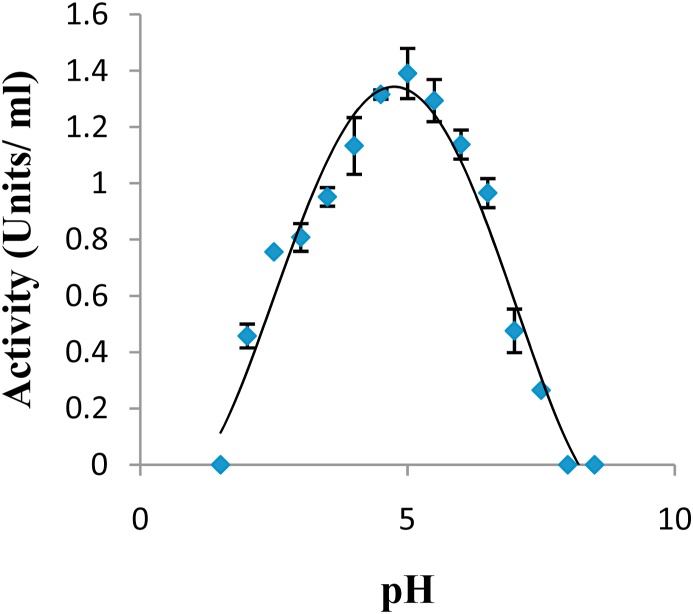
The optimum pH for the purified peroxidase from Moringa oleifera leaves was 5.0 (Figure 6).
Figure 6.
Effect of pH on the activity of purified peroxidase from Moringa oleifera. The optimum pH of the purified enzyme at 30 °C was 5.0.
3.9. Peroxidase as reporter enzyme
When purified peroxidase from Moringa oleifera was cross-linked with BSA and the product separated on calibrated Sephacryl S-300. It gave a single peak with molecular weight of 81.052 kDa signifying the BSA had bonded with the peroxidase forming a single protein possessing peroxidase activity.
3.10. Synthesis of cross-linked protein network
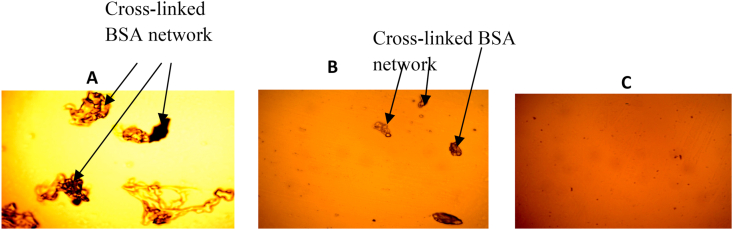
Peroxidase was able to synthesize insoluble fibrous protein from soluble globular BSA in both presence and absence of caffeic acid. However, the presence of 2 mM caffeic acid facilitates the synthesis of more insoluble matrix as seen in Figure 7.
Figure 7.
Photomicrographs of Cross-linked Protein Networks. The synthesis of fibrous protein networks were carried out as earlier described in the presence of peroxidase, caffeic acid, hydrogen peroxide, and BSA at 50 °C for 18 h. Where A is the result obtained when peroxidase catalyzed BSA crosslink in the presence of 2mM caffeic Acid; B is the result obtained when peroxidase catalyzed BSA crosslink without caffeic acid; and C is the result obtained with only BSA and caffeic acid (control).
4. Discussion
We reported in this study a rapid and efficient purification scheme for peroxidase from Moringa oleifera and demonstrated its potential in the synthesis of cross-link protein network and as a reporter enzyme. Activity staining under non-denaturing conditions revealed the presence of only one form of peroxidase. This is consistent with the result obtained from the initial purification of peroxidase from Moringa leaves by Khatun et al. (2012) which reveal the presence of only one form of peroxidase.
Expression of peroxidase in young and matured leaves of Moringa oleifera was found to be 1.3541 ± 0.1002 and 1.4082 ± 0.1278 units/mg protein respectively. Although the matured leaves extract possessed more peroxidase activity than the young leaves, it is however not significant. Previous work on the extraction of peroxidase from Moringa oleifera leaves by Khatun et al. (2012) showed to have a specific activity of 2.11 units/mg protein. This difference may be due to the different substrate used in the individual study. Also, factors such as age, time of harvest, and other environmental conditions which are known to affect peroxidase expression in plants could also be involved.
Aqueous two-phase separation (ATPS) used in this study as the prime purification process was found to be viable, rapid, and efficient combining both purification and concentration of the crude enzymes to about 263% recovery and a purification fold of about 10. The increase in recovery and purification fold utilizing ATPS could be due to partitioning of non-target proteins, natural inhibitors and other contaminants from the desired enzyme to the PEG-rich phase. Thus, depending on the type of biotechnological application, this purification process may be sufficient. Further purification on gel filtration (Sephadex G-100) was able to purify the protein to homogeneity with a recovery of 84% and purification fold of 4. The loss of enzyme recovery and purification suggests that the initial purification step may have been sufficient in purifying the enzyme. Previous work from peroxidase purification from Moringa oleifera by Khatun et al. (2012) used a combination of ammonium sulphate precipitation, DEAE-cellulose column chromatography, Sephadex –G 200 column chromatography, and Con-A column chromatography resulted in 28% recovery and purification fold of 164. When compared with Khatun et al. (2012), the purification process devised for this study is considerably fast, cheap, reliable, and less cumbersome with a higher recovery.
In order to ascertain the purity, integrity, and molecular weight of the purified enzymes, native and subunit molecular weight of the purified enzyme was carried out on calibrated Sephadex G-100 and SDS-PAGE respectively. The purified enzyme appeared to be monomeric with native and subunit molecular weight to be 38.98 ± 1.41 and 43.5 ± 1.63 kDa respectively. This result is consistent with the initial report by Khatun et al. (2012), which reported a monomeric peroxidase from Moringa oleifera with molecular weight of 43kDa. This result is also consistent with the majority of monomeric peroxidases purified from plant having molecular weight between 30-60 kDa (Khatun et al., 2012). However, some exceptions, like kolanut peroxidase have been shown to be dimeric with low molecular weights (Adewale and Adekunle, 2018).
The purified peroxidase was stable over a pH range of 4.0–6.0 with its optimum pH around 5.0 and unstable at alkaline pH. The loss of activity at this alkaline pH may be due to instability of the heme-binding to the enzyme at low pH or as a result of protein denaturation or ionic changes in the heme group at high pH (Adams, 1997). Other studies have reported similar results where most peroxidases from different sources showed optimum activity in the pH range of 4.5–6.5 (Pina et al., 2001; Leon et al., 2002; Deepa and Arumughan, 2002; Diao et al., 2011; Adewale and Adekunle, 2018).
Optimum temperature for the purified peroxidase was 30 °C which is in agreement with the work Civello et al. (1995) who reported maximum enzyme activity at 30 °C for peroxidase purified from strawberry fruits. It is also interesting to note that Diao et al. (2011) reported optimum temperature on four different sources of peroxidase as follows; 40 °C for Allium sativum and Sorghum bicolor and 30 °C for Ipomoea batatas and Raphanus sativu which are consistent with this result. However, Khatun et al. (2012) reported that the optimum temperature for peroxidase from Moringa oleifera was 50 °C this is in sharp contrast to our report. The reason may be due to the purification adopted for this study. For PEG are known to bind to protein and this may have slightly altered the structure of the protein resulting in lower optimum temperature.
Reporter enzymes have immense usage in ELISA kit since it is known to detect antigens and proteins since they are known to trigger the formation of colored product which can be visualized and quantified (Al-Shaban and Abdel-Hamid, 2009). However, horseradish peroxidase is the most widely used as a reporter enzyme because it posseses the ability to produce a chromogenic product at a very low concentration. In this study, we were able to cross-link peroxidase from Moringa oleifera with BSA utilizing glutaraldehyde as the crosslinker, which produced a single activity peak from Sephacyrl S-300 chromatogram with a molecular weight of 81 ± 1.9 kDa. The increase in molecular weight of the protein obtained suggested that peroxidase has fused with BSA to create a new high molecular weight protein possessing peroxidase activity. In other words, cross-linked peroxidase has been used to report BSA, implying it could be useful in reporting other proteins. This is also similar to the result obtained from the cross-linking of peroxidase from kolanut with BSA (Adewale and Adekunle, 2018).
The potentials of Moringa oleifera leave peroxidase as a catalyst in the synthesis of cross-linked protein networks, were further analyzed. Enzymatic cross-linking of proteins has gained increasing interest in food technology to create novel food products or improve textural properties of dairy products and biopolymers based on the cross-linking of the amino side chains such as tyrosine, glutamine, and lysine residues in the proteins, resulting in the formation of a new functional three-dimensional protein network (Chen et al., 2002; Thalmann and Lötzbeyer, 2002) and the traditional enzyme used mainly for this purpose is transglutaminase (Bönisch et al., 2007). The ability of Moringa peroxidase in the effective cross-linking of proteins was achieved in this study. The enzyme could catalyze the formation of fibrous protein networks from soluble proteins (BSA). The cross-linking was carried out in the presence of low molecular weight caffeic acid. This phenolic source acts as a substrate for the cross-linking process. The phenolic substrate is integrated into the polymerizing complex as an interconnection between the single protein molecules for each of the samples. In principle, it is also possible that other amino acid residues of the proteins may have acted as the electrophilic substrate for peroxidase resulting in direct protein-protein crosslinks.
In conclusion, the finding was able to show that peroxidase is abundant in Moringa oleifera leaves and was purified to homogeneity in a two-step purification process thereby considerably reducing cost and time. The purified enzyme possesses some combination of properties that could be useful for biotechnological applications i.e. in the synthesis of cross-linked protein network and as a reporter enzyme.
Declarations
Author contribution statement
Isaac Olusanjo Adewale: Contributed reagents, materials, analysis tools or data.
Oladoyin Grace Famutimi: Analyzed and interpreted the data.
Fatimah Adeola Kadiri, Olakunle Abiodun Kolapo: Performed the experiments.
Oluwadare Joel Agunbiade: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adams J.B. Regeneration and the kinetics of peroxidase inactivation. Food Chem. 1997;60(2):201–206. [Google Scholar]
- Adewale I.O., Adekunle A.T. Biochemical properties of peroxidase from white and red cultivars of kolanut (Cola nitida) Biocatal. Agric. Biotechnol. 2018;14:1–9. [Google Scholar]
- Al-Shaban Z., Abdel-Hamid A. Doctoral dissertation, Univesiti Sains Malaysia; 2009. Comparison between RT-PCR and ELISA for the Detection of HBV in Blood Donors; pp. 1–98. [Google Scholar]
- Ayhan H., Ayhan F., Gülsu A. Highly biocompatible enzyme aggregates crosslinked by L-lysine. Turk. J. Biochem. 2012;37(1):14–20. [Google Scholar]
- Bönisch M.P., Huss M., Weitl K., Kulozik U. Transglutaminase cross-linking of milk proteins and impact on yoghurt gel properties. Int. Dairy J. 2007;17(11):1360–1371. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chen T., Embree H.D., Wu L.Q., Payne G.F. In vitro protein-polysaccharide conjugation: tyrosinase-catalyzed conjugation of gelatin and chitosan. Biopolymers. 2002;64:292–302. doi: 10.1002/bip.10196. [DOI] [PubMed] [Google Scholar]
- Civello P.M., Martinez G.A., Chaves A.R., Anon M.C. Peroxidase from strawberry fruit (Fragaria ananassa Duch.): partial purification and determination of some properties. J. Agric. Food Chem. 1995;43(10):2596–2601. [Google Scholar]
- Dalal S., Gupta M.N. Treatment of phenolic waste water by horseradish peroxidase immobilized by bioaffinity layering. Chemosphere. 2007;67(4):741–747. doi: 10.1016/j.chemosphere.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Deepa S.S., Arumughan C. Purification and characterization of soluble peroxidase from oil palm (Elaeis guineensis Jacq.) leaf. Phytochemistry. 2002;61(5):503–511. doi: 10.1016/s0031-9422(02)00167-x. [DOI] [PubMed] [Google Scholar]
- Diao M., Kone O.H., Ouedraogo N., Bayili R.G., Bassole I.H., Dicko M.H. Comparison of peroxidase activities from Allium sativum, Ipomoea batatas, Raphanus sativus and Sorghum bicolor grown in Burkina Faso. Afr. J. Biochem. Res. 2011;5(4):124–128. [Google Scholar]
- Ezhilarasi A.A., Vijaya J.J., Kaviyarasu K., Maaza M., Ayeshamariam A., Kennedy L.J. Green synthesis of NiO nanoparticles using Moringa oleifera extract and their biomedical applications: cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B Biol. 2016;164:352–360. doi: 10.1016/j.jphotobiol.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Jadhav U.U., Dawkar V.V., Telke A.A., Govindwar S.P. Decolorization of Direct Blue GLL with enhanced lignin peroxidase enzyme production in Comamonas sp UVS. J. Chem. Technol. Biotechnol. 2009;84(1):126–132. [Google Scholar]
- Kay E., Shannon L.M., Lew J.Y. Peroxidase isozymes from horseradish roots II. Catalytic properties. J. Biol. Chem. 1967;242(10):2470–2473. [PubMed] [Google Scholar]
- Khatun S., Ashraduzzaman M., Karim M.R., Pervin F., Absar N., Rosma A. Purification and characterization of peroxidase from Moringa oleifera L. leaves. BioResources. 2012;7(3):3237–3251. [Google Scholar]
- Kim Y.H., Yoo Y.J. Peroxidase production from carrot hairy root cell culture. Enzym. Microb. Technol. 1996;18(7):531–535. [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai Y.C., Lin S.C. Application of immobilized horseradish peroxidase for the removal of p-chlorophenol from aqueous solution. Process Biochem. 2005;40(3-4):1167–1174. [Google Scholar]
- Leon J.C., Alpeeva I.S., Chubar T.A., Galaev I.Y., Csoregi E., Sakharov I.Y. Purification and substrate specificity of peroxidase from sweet potato tubers. Plant Sci. 2002;163(5):1011–1019. [Google Scholar]
- Leone A., Spada A., Battezzati A., Schiraldi A., Aristil J., Bertoli S. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int. J. Mol. Sci. 2015;16(6):12791–12835. doi: 10.3390/ijms160612791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onsa G.H., Bin Saari N., Selamat J., Bakar J. Purification and characterization of membrane-bound peroxidases from Metroxylon sagu. Food Chem. 2004;85(3):365–376. [Google Scholar]
- Pina D.G., Shnyrova A.V., Gavilanes F., Rodríguez A., Leal F., Roig M.G., Sakharov I.Y., Zhadan G.G., Villar E., Shnyrov V.L. Thermally induced conformational changes in horseradish peroxidase. Eur. J. Biochem. 2001;268(1):120–126. doi: 10.1046/j.1432-1033.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Popoola O.J., Obembe O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013;150(2):682–691. doi: 10.1016/j.jep.2013.09.043. [DOI] [PubMed] [Google Scholar]
- Rodrigo C., Rodrigo M., Alvarruiz A., Frigola A. Thermal inactivation at high temperatures and regeneration of green asparagus peroxidase. J. Food Protect. 1996;59(10):1065–1071. doi: 10.4315/0362-028X-59.10.1065. [DOI] [PubMed] [Google Scholar]
- Saitou T., Kamada H., Harada H. Isoperoxidase in hairy roots and regenerated plants of horseradish (Armoracia lapathifolia) Plant Sci. 1991;75(2):195–201. [Google Scholar]
- Sakai S., Khanmohammadi M., Khoshfetrat A.B., Taya M. Horseradish peroxidase-catalyzed formation of hydrogels from chitosan and poly (vinyl alcohol) derivatives both possessing phenolic hydroxyl groups. Carbohydr. Polym. 2014;111:404–409. doi: 10.1016/j.carbpol.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Saunders B.C. Peroxidases and catalases. In: Eichorn G.L., editor. Inorganic Biochemistry. Elsevier; Amsterdam: 1973. pp. 988–1021. [Google Scholar]
- Shigeto J., Tsutsumi Y. Diverse functions and reactions of class III peroxidases. New Phytol. 2016;209(4):1395–1402. doi: 10.1111/nph.13738. [DOI] [PubMed] [Google Scholar]
- Sivasankari B., Anandharaj M., Gunasekaran P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J. Ethnopharmacol. 2014;153:408–423. doi: 10.1016/j.jep.2014.02.040. [DOI] [PubMed] [Google Scholar]
- Srinivas N.D., Barhate R.S., Raghavarao K.S.M.S. Aqueous two-phase extraction in combination with ultrafiltration for downstream processing of Ipomoea peroxidase. J. Food Eng. 2002;54(1):1–6. [Google Scholar]
- Thalmann C., Lötzbeyer T. Enzymatic cross-linking of proteins with tyrosinase. Eur. Food Res. Technol. 2002;214(4):276–281. [Google Scholar]
- Van Huystee R.B., Cairns W.L. Progress and prospects in the use of peroxidase to study cell development. Phytochemistry. 1982;21(8):1843–1847. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.