FIGURE 1.
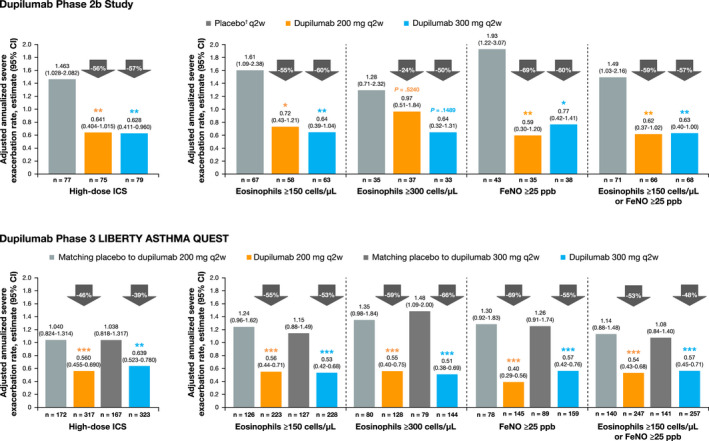
Annualized rate of severe exacerbations in dupilumab‐treated patients (q2w) vs placebo during the 24‐wk treatment period in the phase 2b study and 52‐wk treatment period in the phase 3 QUEST study on high‐dose ICS at baseline and further stratified by baseline eosinophil and FeNO levels. †In the phase 2b study, the same amount of placebo was given regardless of dupilumab dose (not volume‐matched as in the phase 3 QUEST study). ***P < .001, **P < .01, *P < .05 vs placebo. CI, confidence interval; FeNO, fractional exhaled nitic oxide; q2w, every 2 wk
