FIGURE 2.
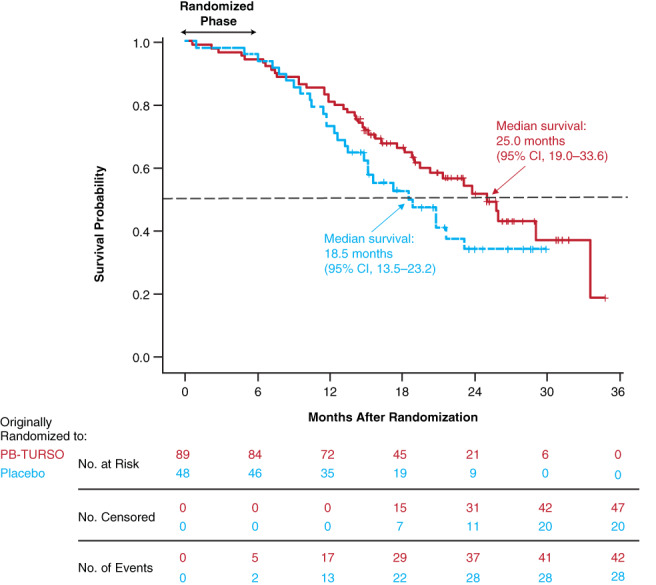
Overall survival in the entire randomized population. Starting at the conclusion of the randomized phase at month 6 (24 weeks), eligible participants could enroll in the OLE. Of 98 eligible participants, 90 (92%) continued on in the OLE, 34 of whom were originally randomized to placebo and 56 to active drug. The survival analysis encompassed all participants randomized at time 0, including those who did not enter the OLE, discontinued study participation, or were lost to follow‐up. The hazard ratio for death in the group originally randomized to active treatment was 0.56 (P = .023 vs group originally randomized to placebo). OLE, open‐label extension; PB‐TURSO, sodium phenylbutyrate‐taurursodiol
