Fig. 5.
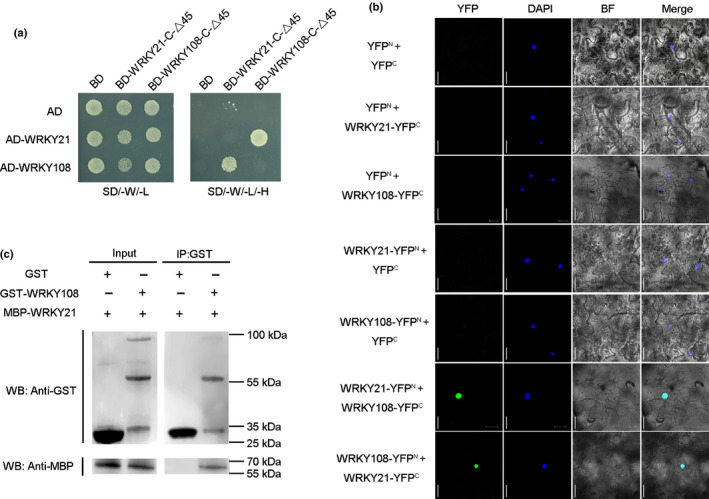
WRKY21 physically interacts with WRKY108. (a) Y2H assay for the interaction of WRKY21 and WRKY108. WRKY21 and WRKY108 were fused to GAL4 activation domain to generate AD‐WRKY21 and AD‐WRKY108. WRKY21‐C‐△45 (described in Supporting Information Fig. S5a) and WRKY108‐C‐△45 (described in Fig. S5b) were fused to GAL4 binding domain to generate BD‐WRKY21‐C‐△45 and BD‐WRKY108‐C‐△45. Yeast cells co‐transformed with AD‐WRKY21/BD‐WRKY21‐C‐△45, AD‐WRKY21/ BD‐WRKY108‐C‐△45, AD‐WRKY108/BD‐WRKY21‐C‐△45 and AD‐WRKY108/BD‐WRKY108‐C‐△45 were grown on selective media SD/−W/−L and SD/−W/−L/−H. Co‐expression of AD/BD, AD/BD‐WRKY21‐C‐△45, AD/BD–WRKY108‐C‐△45, AD‐WRKY21/BD and AD‐WRKY108/BD were used as negative controls. SD/−W/−L, (−Trp−Leu); SD/−W/−L/−H, (−Trp−Leu−His). (b) BiFC analysis for the interaction between WRKY21 and WRKY108. N‐ and C‐terminal fragments of yellow fluorescent protein (YFP) (YFPN and YFPC) were fused to the C terminus of WRKY21 and WRKY108, respectively. Green signals indicate YFP, and the blue signals indicate the cell nucleus that was specifically stained by 4′,6‐diamidino‐2‐phenylindole (DAPI). Bars, 20 μm. (c) GST pull‐down assay for interaction between WRKY21 and WRKY108 in vitro. MBP‐WRKY21 and GST‐WRKY108 were expressed and purified in E. coli and subjected to GST pull‐down assays. GST/GST‐WRKY108 and MBP‐WRKY21 proteins were detected by immunoblotting using anti‐GST and anti‐MBP antibodies, respectively.
