Fig. 4.
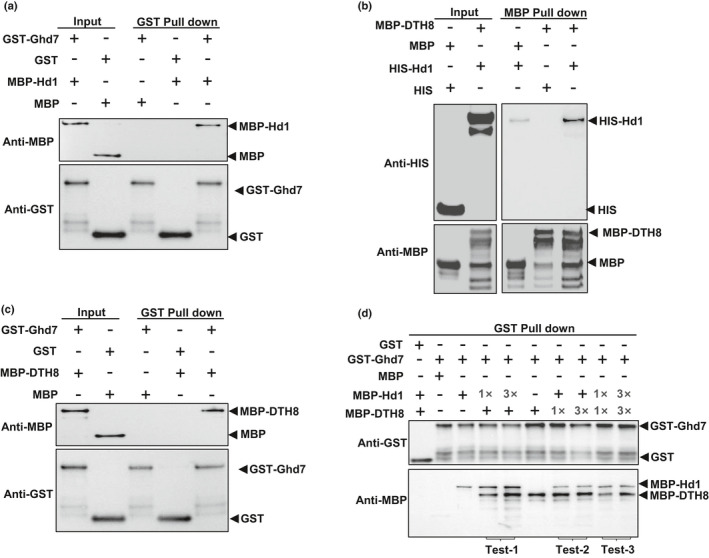
In vitro pulldown assays for interactions between and among Hd1, Ghd7 and DTH8. (a) Hd1 interacted with Ghd7. GST‐Ghd7 is used as bait. (b) Hd1 interacted with DTH8. MBP‐DTH8 is used as bait. (c) DTH8 interacted with Ghd7. GST‐Ghd7 is used as bait. (d) Interactions among Hd1, Ghd7 and DTH8. GST‐Ghd7 was used as a bait. In Test‐1, the prey MBP‐Hd1 protein was first added to the bait GST‐Ghd7. From 1× to 3×, the intensity of the anti‐MBP hybridization band of MBP‐Hd1 gradually increased, indicating that more MBP‐Hd1 was pulled down by GST‐Ghd7. After washing, the second prey, MBP‐DTH8, was added, but the amount was constant. The intensity of the hybridization band of MBP‐DTH8 was also increased, which implies that DTH8 was more likely to bind the increased Hd1, proving the formation of a three‐protein complex of Ghd7/Hd1/DTH8. In Test‐2, the prey MBP‐DTH8 was added first to the bait GST‐Ghd7. From 1× to 3×, the anti‐MBP hybridization band of MBP‐DTH8 remained unchanged, indicating that 1× of DTH8 was enough to bind to all the bait GST‐Ghd7. After washing, the second prey MBP‐Hd1 was added with a constant amount. Its hybridization band was basically unchanged, implying that Hd1 bound to Ghd7 and DTH8 with a different domain, forming a three‐protein complex. In Test‐3, 1× of prey MBP‐DTH8 and MBP‐Hd1, or 3× of prey MBP‐DTH8 and MBP‐Hd1 were added simultaneously to the reaction. It was observed that the hybridization bands of MBP‐DTH8 and MBP‐Hd1 had similar intensities. Test‐1, Test‐2 and Test‐3 together explained that Ghd7/DTH8/Hd1 formed a three‐protein complex at a ratio of 1 : 1 : 1, instead of independent pairwise interactions. It is not the case that the amount of GST‐Ghd7 was excessive, and thus a part of it bound to MBP‐Hd1 and another part of it interacted with MBP‐DTH8.
