Figure 4.
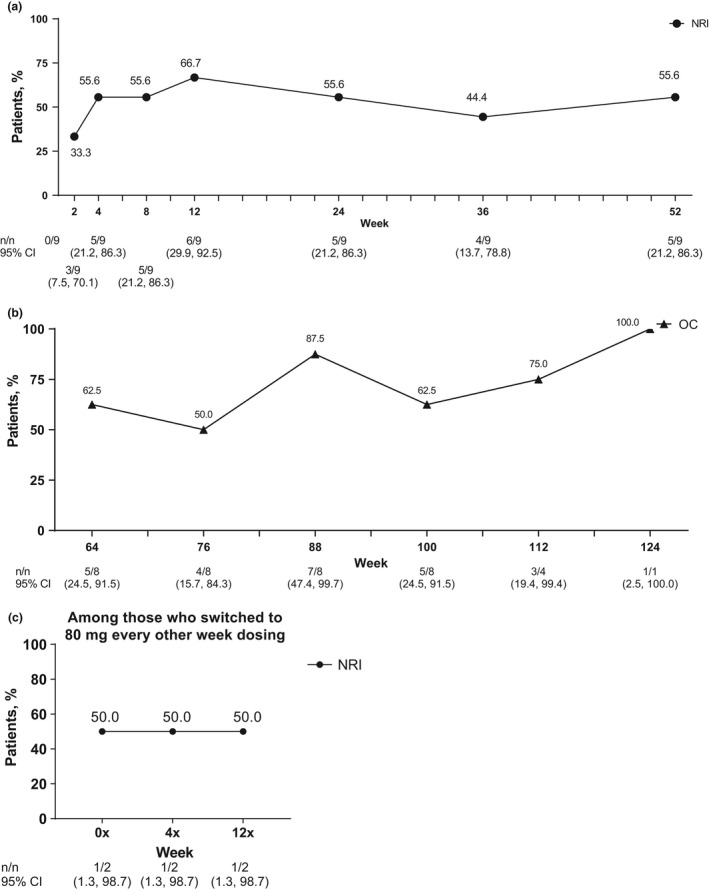
NRS30 response rate among patients with baseline Numeric Rating Scale (NRS) of 3 or more (at worst) among patients (a) treated with weekly adalimumab (ADA) 40 mg through 52 weeks, (b) treated with weekly ADA 40 mg from 64 through 124 weeks and (c) who switched to ADA 80 mg every other week. Missing data were handled using (a,c) the non‐responder imputation (NRI) method or (b) data are shown as observed cases (OC). NRS30, 30% or more reduction and 1 unit or more reduction from baseline in Patient’s Global Assessment for Skin Pain NRS.
