Abstract
Objectives
Recent reports suggest an association between the inflammatory response after aneurysmal subarachnoid haemorrhage (aSAH) and patients' outcome. The primary aim of this study was to identify a potential association between the inflammatory response after aSAH and 1‐year outcome. The secondary aim was to investigate whether the inflammatory response after aSAH could predict the development of delayed cerebral ischaemia (DCI).
Materials and methods
This prospective observational pilot study included patients with an aSAH admitted to Sahlgrenska University Hospital, Gothenburg, Sweden, between May 2015 and October 2016. The patients were stratified according to the extended Glasgow Outcome Scale (GOSE) as having an unfavourable (score: 1–4) or favourable outcome (score: 5–8). Furthermore, patients were stratified depending on development of DCI or not. Patient data and blood samples were collected and analysed at admission and after 10 days.
Results
Elevated serum concentrations of inflammatory markers such as tumour necrosis factor‐α and interleukin (IL)‐6, IL‐1Ra, C‐reactive protein and intercellular adhesion molecule‐1 were detected in patients with unfavourable outcome. When adjustments for Glasgow coma scale were made, only IL‐1Ra remained significantly associated with poor outcome (p = 0.012). The inflammatory response after aSAH was not predictive of the development of DCI.
Conclusion
Elevated serum concentrations of inflammatory markers were associated with poor neurological outcome 1‐year after aSAH. However, inflammatory markers are affected by many clinical events, and when adjustments were made, only IL‐1Ra remained significantly associated with poor outcome. The robustness of these results needs to be tested in a larger trial.
Keywords: delayed cerebral ischaemia, inflammation, outcome, subarachnoid haemorrhage
1. INTRODUCTION
Aneurysmal subarachnoid haemorrhage (aSAH) is defined as the spontaneous rupture of a cerebral aneurysm that causes a rapid flow of blood into the subarachnoid space, with an annual global incidence of approximately 7–10/100,000 individuals. The estimated mortality is around 40%. A third of the patients who survive the initial haemorrhage will develop secondary injuries. 1 , 2 Known risk factors for poor outcome after aSAH (death or low functional status) are large amounts of blood in the first radiographic examination, poor neurological status at admission, the development of delayed cerebral ischaemia (DCI), hydrocephalus and development of an infectious complication. 3 , 4 , 5 , 6 , 7
Of these, DCI is the leading cause for mortality and morbidity after aSAH. Previously, DCI was considered to be caused exclusively by vasospasm in cerebral arteries. 2 However, recent studies have revealed a more complex scenario wherein vasospasm, a global reduction in blood flow, microcirculatory vasoconstriction, micro‐thrombosis, cortical spreading depolarizations, blood–brain barrier dysfunction and inflammation is considered to contribute to the development of DCI. 8 , 9 , 10 , 11 , 12 , 13
Furthermore, studies have identified associations between the elevated concentrations of inflammatory markers in the peripheral blood and poor outcome after aSAH. 14 , 15 , 16 , 17 , 18 , 19 In general, these studies have assessed the outcome of patients during the first few months after ictus. However, some reports suggest that patients may experience continued long‐term neurological improvements or deterioration, 20 motivating an extended assessment of the association between inflammation and outcome after aSAH.
Inflammatory markers, such as the cytokines tumour necrosis factor‐α (TNF‐α), interleukin (IL)‐ 6, IL‐1Ra, intracellular adhesion molecule‐1 (ICAM‐1) and the acute‐phase protein C‐reactive protein (CRP), have previously been associated with a poor short‐term outcome after aSAH. 15 , 16 , 21 , 22 However, these markers are also associated with other risk factors for poor prognosis such as infection and ischaemia after aSAH. 23 Although elevated levels of inflammatory markers have also been linked to the development of DCI after aSAH, the existing evidence is conflicting. 17 , 24 , 25 , 26 , 27 Very few reports have explored the association between inflammatory markers seen after aSAH and long‐term outcome of patients.
Our hypothesis was that poor long‐term outcome 1 year after aSAH could be predicted by elevated levels of TNF‐α, IL‐6, IL‐1Ra, ICAM‐1 and CRP but also that the development of DCI could be predicted by elevated levels of these markers.
1.1. Aim
The primary aim of this pilot study was to explore the potential association between elevated levels of TNF‐α, IL‐6, IL‐1Ra, ICAM‐1 and CRP in patients with aSAH and 1‐year outcome. The secondary aim was to investigate whether elevated levels of TNF‐α, IL‐6, IL‐1Ra, ICAM‐1 and CRP could predict the development of DCI.
2. METHODS
2.1. Study design
This prospective observational pilot study adheres to ‘Strengthening the reporting of observational studies in epidemiology’ (STROBE) guidelines. 28
2.2. Study population
Patients with aSAH admitted to Sahlgrenska University Hospital, Gothenburg, Sweden between May 2015 and October 2016 were screened for eligibility for study enrolment. The inclusion criteria were age ≥18 years and the verification of aSAH by digital subtraction angiography. The exclusion criteria were a history of previous stroke or brain injury. The patients were treated in the neurointensive care unit in accordance with a local protocol, mainly consistent with the American Heart Association/American Stroke Association guidelines. 29 The aneurysms were secured as early as possible, within 24 h after admission. Prophylactic Nimodipine (Nimotop®, 0.2 mg/ml; Bayer, Leverkusen, Germany) was administered intravenously. Patients who developed hydrocephalus received an external ventricular drain, and those developing signs of infection were treated with antibiotics, fluids and vasopressors as needed. Patients who developed clinical or radiological signs of DCI underwent hemodynamic optimization and angiography to determine the possibility of intra‐arterial nimodipine administration or balloon angioplasty.
2.3. Data collection
The patients' medical history, imaging results and interventions during the study period were collected prospectively. Day 0 was defined as the day of admission.
Clinical condition was scored on hospital admission by the attending neurosurgeon according to the World Federation of Neurological Surgeons scale. 30 The Glasgow coma scale (GCS) 31 score was rated at the emergency department of the primary hospital.
The amount of blood in the subarachnoid space was evaluated and scored according to the modified Fisher's scale score. 32
Infection was defined as clinical signs of infection in combination with a positive culture from blood, cerebrospinal fluid or tracheal secretion warranting antimicrobial treatment.
Neurological assessments were performed by specially trained nurses at least three times daily. Computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed at the discretion of the attending physician. DCI was defined according to current guidelines as stated by Vergouwen et al. 3
The first set of patient data and blood samples were collected as soon as possible after study inclusion (sample 1) and again at 10 days after admission (sample 2). The samples were drawn from an arterial cannula or a central venous line. The samples were immediately frozen at −80°C until further analysis.
The concentrations of cytokines in blood serum samples were assessed using the Bio‐Plex Pro Human Cytokine 27‐Plex Group I (Bio‐Rad, Hercules, CA, USA; catalogue # M500KCAFOY), MIG (Bio‐Rad; catalogue # 171B6015 M), Bio‐Plex Pro Human TGF‐β 3‐Plex (Bio‐Rad; catalogue # 171W4001 M) and Bio‐Plex Pro Human ICAM‐1/VCAM 2‐Plex sets (Bio‐Rad; catalogue # 171B6009 M/171B6022 M). The samples were then analysed using the Luminex 200 system (Bio‐Rad) according to the manufacturer's instructions. All sample preparation and dilution protocols were performed according to the manufacturer's recommendations. Analytes in which the concentration of the measured factor was below the detection limit were handled by multiple imputation, wherein the value was set as 1/8 of the detection limit of the standard curve. We chose for practical reasons, to use an assay panel which included the factors of interest, mentioned above. All patient data and inflammatory markers included in the analyses are presented in Appendix S1A. CRP and LPK were analysed at the accredited university hospital laboratory.
The functional outcome at 1 year after aSAH was assessed via telephone interview using the extended Glasgow Outcome Scale (GOSE). 33 On this eight‐point scale, a score of 1 represents death, while a score of 8 indicates full recovery. The scale gives a general indication of functional recovery. As our primary end‐point, we chose GOSE at 1 year after ictus. We defined poor outcome as GOSE 1‐4, which include death and severe disability, and favourable outcome as GOSE 5‐8, including moderate disability to full recovery. The full content of GOSE is presented in Appendix S1B.
2.4. Ethics and data sharing
The study adheres to the Declaration of Helsinki, and the study protocol was approved by the Ethical Regional Board of Gothenburg, Sweden (053‐15, T 213‐18). Written informed consent was obtained from each patient or their next of kin before study inclusion. Data may be shared on reasonable request.
2.5. Statistical analysis
Univariable comparisons between groups (GOSE 1–4 vs. 5–8 and DCI vs. non‐DCI) were performed using Fisher's exact test for dichotomous variables and Mann–Whitney U test for continuous/ordered variables.
Areas under the received operating characteristic (ROC) curves were compared using the nonparametric approach of DeLong. 34
Multiple logistic regression was used for multivariable analysis. Due to the limited number of unfavourable outcomes, both regarding GOSE 1–4 and DCI, it was not considered justifiable to include more than two independent variables in the same model at the same time. Furthermore, there was a strong correlation between IL‐1Ra and IL‐6 and thus no model with both these markers included was calculated.
All tests were two‐sided and p‐values below 0.05 were considered statistically significant. SAS for Windows version 9.4 was used for all analysis.
3. RESULTS
A total of 105 patients presented with aSAH at Sahlgrenska University Hospital, during the study period. Of these patients, 76 were screened to determine their eligibility, and 64 were selected for study inclusion. Ultimately, data and blood samples were collected from 58 patients. For the inclusion process, see consort chart in Figure 1. Sixteen patients (28%) developed DCI according to the current definition. 3 Of the 16 patients that developed DCI, median (IQR) GOSE was 5.5 (4.5‐7.5) 1 year after ictus, vs. 5 (4‐7) in the non‐DCI group (p = 0.83). Overall, 30% of the patients had an unfavourable (GOSE 1–4), at 1 year after the aSAH. Four of the 58 patients died, yielding a 1‐year mortality rate of 7% in this cohort. Two additional patients were lost to follow‐up but remained alive according to administrative records. Patient characteristics for the whole study population are shown in Table 1.
FIGURE 1.
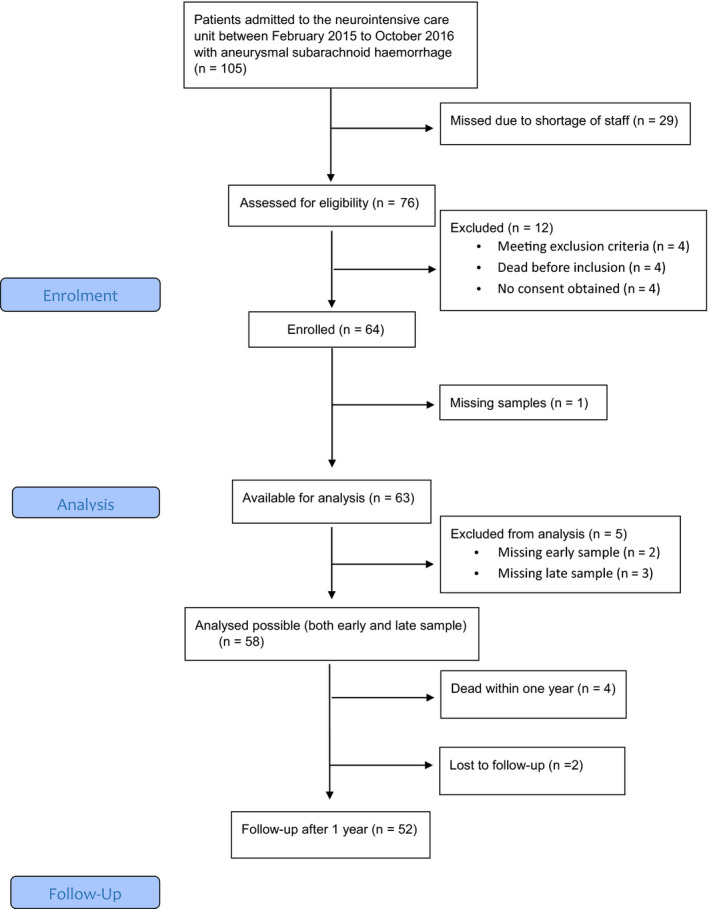
Consort flow chart
TABLE 1.
Patient characteristics
| All patients (n = 58) | Unfavourable GOSE 1–4 (n = 17) |
Favourable GOSE 5–8 (n = 39) |
DCI (n = 16) |
Non–DCI (n = 42) |
|
|---|---|---|---|---|---|
| Sex (female) | 44 (76) | 14 (82) | 28 (72) | 10 (63) | 34 (81) |
| Age (years) | 57 (48–64) | 58 (54–65) | 56 (48–64) | 56 (48–60) | 58 (48–65) |
| Death first year | 4 (7) | 4 (24) | 0 (0) | 1 (6) | 3 (7) |
| Cardiovascular disease | 4 (7) | 2 (12) | 1 (3) | 1 (6) | 3 (7) |
| Hypertension | 20 (34) | 6 (35) | 14 (36) | 4 (25) | 16 (38) |
| Diabetes | 3 (5) | 2 (12) | 1 (3) | 0 (0) | 3 (7) |
| Smoking | 9 (16) | 1 (6) | 8 (21) | 3 (19) | 6 (42) |
| WFNS 1–3 | 40 (69) | 7 (41) | 31 (79) | 10 (63) | 30 (71) |
| WFNS 4‐5 | 18 (31) | 10 (59) | 8 (21) | 6 (38) | 12 (29) |
| GCS 13–15 | 40 (69) | 7 (41) | 31 (79) | 10 (63) | 30 (71) |
| GCS 9–12 | 7 (12) | 3 (18) | 4 (10) | 3 (19) | 4 (10) |
| GCS 3–8 | 11 (19) | 7 (41) | 4 (10) | 3 (19) | 8 (19) |
| Modified Fischer Grade 0–2 | 4 (7) | 0 (0) | 4 (10) | 0 (0) | 4 (10) |
| Modified Fischer Grade 3–4 | 54 (93) | 17 (100) | 35 (90) | 16 (100) | 38 (90) |
|
Aneurysm location Anterior circulation |
44 (76) | 15 (88) | 27 (69) | 15 (94) | 29 (69) |
| Posterior circulation | 14 (24) | 2 (12) | 12 (31) | 1 (6) | 13 (31) |
| Ventricular drainage | 29 (50) | 14 (82) | 15 (38) | 9 (56) | 20 (48) |
| Embolization | 42 (72) | 7 (41) | 34 (87) | 14 (88) | 28 (67) |
| Surgery | 16 (28) | 10 (59) | 5 (13) | 2 (12) | 14 (33) |
| Infection (All) | 24 (41) | 13 (76) | 11 (28) | 11 (69) | 13 (31) |
| Ventriculitis (positive cerebrospinal culture) | 2 (3) | 2 (12) | 0 (0) | 0 (0) | 2 (5) |
| Positive blood culture | 3 (5) | 1 (6) | 2 (5) | 3 (19) | 0 (0) |
| Ventilator associated pneumonia (positive sputum culture) | 16 (28) | 9 (53) | 7 (18) | 8 (50) | 8 (19) |
| Positive urine culture | 1 (2) | 1 (6) | 0 (0) | 0 (0) | 1 (2) |
Number (per cent) or median with interquartile ranges. Infection was defined as clinical signs of infection in combination with a positive culture from blood, cerebrospinal fluid or tracheal secretion warranting antimicrobial treatment.
Two patients were lost to follow‐up, making the total patients with scored GOSE n = 56.
Abbreviations: GCS, Glasgow Coma scale; WFNS, World Federation of Neurosurgical Societies.
3.1. GOSE
3.1.1. Sample 1
A lower GCS rating and a higher Fisher score on admission were associated with poor long‐term outcome (GOSE 1–4). The concentrations of TNF‐α, IL‐6, IL1‐Ra, CRP and ICAM‐1 in sample 1 were significantly elevated in patients with an unfavourable outcome relative to those in patients with a favourable outcome, Table 2. ROC curves that display the unadjusted association between the individual inflammatory markers in sample 1 and poor long‐term outcome are shown in Figure 2. There was no significant difference between the areas under the ROC curves for the five biomarkers. When adjusted for GCS, the association between the inflammatory markers of interest and poor long‐term outcome remained statistically significant for IL‐1Ra but none of the other markers. When adjusted for the modified Fisher scale score, the association remained statistically significant for all the markers except for ICAM‐1, Table 3.
TABLE 2.
Patient data and sample 1—GOSE
| Unfavourable GOSE 1–4 (n = 17) | Favourable GOSE 5–8 (n = 39) | p | |
|---|---|---|---|
| Sex (female) | 14 (82) | 28 (72) | 0.51 |
| Age (years) | 58 (54–64) | 56 (48–64) | 0.28 |
| GCS | 11 (7–13) | 14 (13–15) | 0.01 |
| Modified Fischer Grade 0–2 | 0 (0) | 4 (10) | 0.02 |
| Modified Fischer Grade 3–4 | 17 (100) | 35 (90) | 0.02 |
| IL‐1Ra | 369 (187–524) | 155 (87–253) | 0.007 |
| IL‐6 | 3.30 (0.45–6.93) | 0.45 (0.06–2.92) | 0.01 |
| TNF–α | 23.2 (18.2–29.5) | 18.2 (15.1–20.9) | 0.02 |
| ICAM−1 | 178082 (144650–235454) | 138668 (87755–190669) | 0.05 |
| CRP | 49 (15–73) | 7 (3–20) | 0.01 |
| WCC (1/0) a | 12.7 (10.4–16.0) | 11.2 (8.9–14.1) | 0.23 |
| Platelets (1/0) a | 220 (180–252) | 251 (199–297) | 0.09 |
| Temperature (1/0) a | 38.2 (37.6–38.7) | 37.9 (37.4–38.2) | 0.14 |
Number (per cent) or median with interquartile ranges. IL‐1Ra, IL‐6, TNF‐α, ICAM‐1 concentrations in picogram/L, CRP in mg/L, WCC and platelets count 109/L, temperature in degrees Celsius.
Two patients were lost to follow‐up, making the total patients with scored GOSE n = 56.
Abbreviations: CRP, C‐reactive protein; ICAM, intracellular adhesion molecule; IL‐1Ra, interleukin‐1 receptor antagonist; IL‐6, interleukin 6, TNF‐α, tumour necrosis factor alpha; WCC, white cell count.
Number of missing in the two groups, respectively.
FIGURE 2.
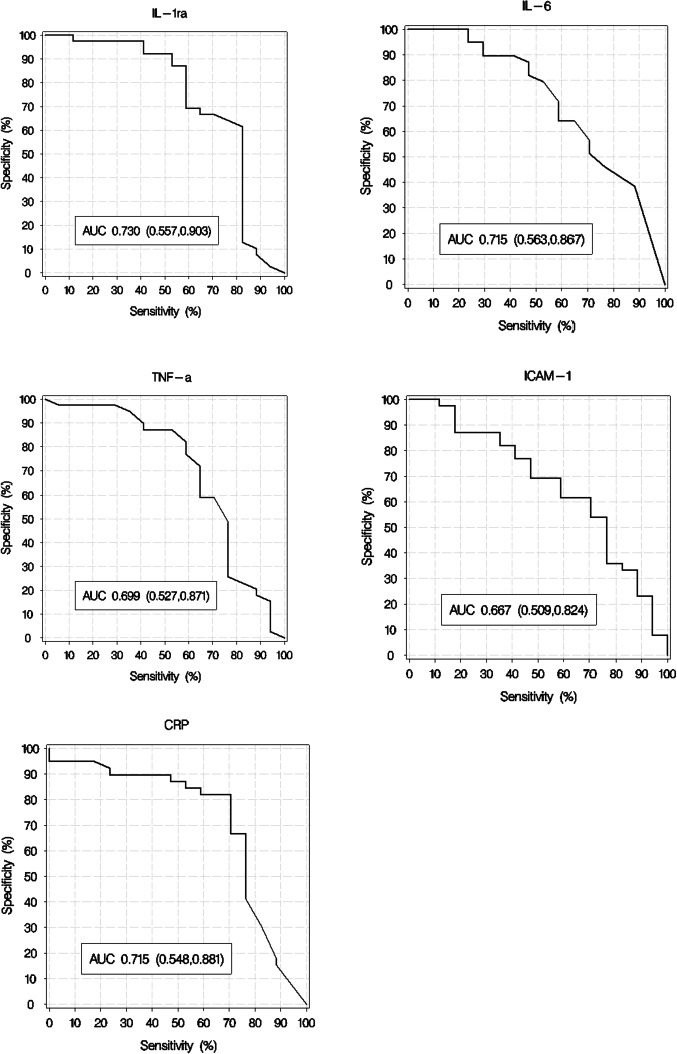
Received operating characteristic curve. ROC curves, sample 1, for IL‐1Ra, IL‐6, TNF‐α; ICAM‐1 and CRP showing their unadjusted association to poor outcome (GOSE 1‐4). IL‐1Ra; interleukin‐1 receptor antagonist, IL; interleukin, TNF‐α; tumour necrosis factor alpha, ICAM; intracellular adhesion molecule, CRP; C‐reactive protein
TABLE 3.
Multivariate analysis
| Unadjusted | Adjusted for GCS | Adjusted for modified Fischer scale | |
|---|---|---|---|
| Sample 1 | |||
| IL‐1Ra | 0.0065 | 0.012 | 0.0068 |
| IL‐6 | 0.0099 | 0.34 | 0.012 |
| TNF‐a | 0.018 | 0.13 | 0.028 |
| ICAM‐1 | 0.049 | 0.26 | 0.055 |
| CRP | 0.011 | 0.22 | 0.020 |
| Sample 2 | |||
| CRP | 0.015 | 0.10 | 0.0099 |
p‐values for multivariate analyses for unfavourable outcome GOSE 1–4.
Abbreviations: CRP, C‐reactive protein; ICAM, intracellular adhesion molecule; IL, interleukin; IL‐1Ra, interleukin‐1 receptor antagonist; TNF‐α, tumour necrosis factor alpha.
Due to a strong collinearity between IL‐1Ra and IL‐6, it was not justifiable to include both these variables in the same statistical model. Performing analysis from sample 1 of all pairs of the five biomarkers (except for the pair of IL‐1Ra and IL‐6) only IL‐1Ra remained significant in all its combinations.
3.1.2. Sample 2
At the second sample time point, development of hydrocephalus treated with ventricular drainage, culture verified infection and an elevated level of CRP was significantly associated with a poor long‐term outcome. No additional significant differences in cytokine concentrations in sample 2 were observed between patients with favourable and unfavourable outcome, Table 4. When adjusted for presence hydrocephalus or infection, no statistically significant association between CRP and outcome was found.
TABLE 4.
Patient data and sample 2—GOSE
| Unfavourable GOSE 1–4 (n = 17) | Favourable GOSE 5–8 (n = 39) | p | |
|---|---|---|---|
| Hydrocephalus | 14 (82) | 61 (41) | 0.008 |
| Infection | 13 (76) | 11 (28) | 0.001 |
| IL‐1Ra | 157 (124–295) | 157 (106–275) | 0.68 |
| IL‐6 | 0.78 (0.06–1.42) | 0.10 (0.06–2.04) | 0.35 |
| TNF‐α | 20.9 (16.6–25.2) | 18.2 (13.7–20.9) | 0.23 |
| ICAM‐1 | 166606 (140480–216188) | 164875 (126551–193014) | 0.57 |
| CRP (0/1) a | 36 (20–71) | 10 (6–39) | 0.02 |
| WCC (0/8) a | 10.8 (10.3–12.5) | 10.4 (8.6–12.6) | 0.24 |
| Platelets (2/7) a | 266 (215–343) | 300 (267–390) | 0.39 |
| Temperature (2/8) a | 38.1 (38.0–38.6) | 37.9 (37.5–38.7) | 0.36 |
Number (per cent) or median with interquartile ranges. IL‐1Ra, IL‐6, TNF‐α, ICAM‐1 concentrations in picogram/litre, CRP in mg/l, WCC and platelets count 109/L, temperature in degrees Celsius.
Two patients were lost to follow‐up, making the total patients with scored GOSE n = 56.
Abbreviations:CRP, C‐reactive protein; ICAM, Intracellular Adhesion Molecule; IL‐1Ra, Interleukin‐1 receptor antagonist; IL‐6, Interleukin 6, TNF‐α, Tumour necrosis factor alpha; WCC, White cell count.
Number of missing in the two groups, respectively.
3.2. DCI
3.2.1. Sample 1
Patients that developed DCI had lower GCS at admission, although the difference did not reach statistical significance (p = 0.08). Patients developing DCI all had a more severe aSAH with modified Fisher score of 3 or 4. No significant differences were observed in the serum concentrations of clinically relevant inflammatory markers, between the DCI and non‐DCI cases in sample 1, Table 5. Accordingly, the levels of inflammatory markers at admission were not found to be associated with the development of DCI in our cohort.
TABLE 5.
Patient data and sample 1—DCI
| DCI (n = 16) | Non‐DCI (n = 42) | p | |
|---|---|---|---|
| Sex (female) | 10 (62) | 34 (81) | 0.18 |
| Age (years) | 56 (48–60) | 58 (48–65) | 0.69 |
| GCS | 13 (10–14) | 14 (12–15) | 0.08 |
| Modified Fischer Grade 0–2 | 0 (0) | 4 (9) | 1.00 |
| Modified Fischer Grade 3–4 | 16 (100) | 38 (90) | 1.00 |
| IL‐1Ra | 197 (87–366) | 177 (124–275) | 0.82 |
| IL‐6 | 2.92 (0.08–3.67) | 1.08 (0.06–3.30) | 0.42 |
| TNF‐α | 20.7 (15.4–23.8) | 18.2 (15.6–22.4) | 0.90 |
| ICAM‐1 | 132951 (100732–179923) | 163054 (110488–206250) | 0.28 |
| CRP | 14 (5–48) | 8 (3–29) | 0.38 |
| WCC (0/1) a | 10.2 (8.8–12.2) | 12.7 (9.9–16.4) | 0.05 |
| Platelets (0/1) a | 236 (207–293) | 248 (197–292) | 0.78 |
| Temperature (0/1) a | 38.2 (37.6–38.4) | 37.7 (37.4–38.2) | 0.14 |
Number (per cent) or median with interquartile ranges.IL‐1Ra, IL‐6, TNF‐α, ICAM‐1 concentrations in picogram/litre, CRP in mg/l, WCC and platelets count 109/L, temperature in degrees Celsius. IL‐1Ra, Interleukin‐1 receptor antagonist, IL‐6, Interleukin 6, TNF‐α, Tumour necrosis factor alpha,
Abbreviations: CRP, C‐reactive protein; ICAM, intracellular adhesion molecule; WCC, white cell count.
Number of missing in the two groups, respectively.
3.2.2. Sample 2
Culture‐verified infection and elevated temperature, but not development of hydrocephalus at sample 2, were significantly associated with development of DCI. No significant differences were observed in the serum concentrations of clinically relevant inflammatory markers, between the DCI and non‐DCI cases in sample 2, Table 6.
TABLE 6.
Patient data and sample 2—DCI
| DCI (n = 16) | Non‐DCI (n = 42) | p | |
|---|---|---|---|
| Hydrocephalus | 9 (56) | 21 (50) | 0.77 |
| Infection | 11 (69) | 13 (31) | 0.02 |
| IL‐1Ra | 165 (115–316) | 155 (106–275) | 0.43 |
| IL‐6 | 0.28 (0.06–1.08) | 0.28 (0.06–1.74) | 0.76 |
| TNF‐α | 18.1 (15.4–23.8) | 18.2 (15.1–23.2) | 0.86 |
| ICAM‐1 | 150789 (137488–174421) | 174005 (126687–210409) | 0.26 |
| CRP (0/1) | 24 (8–89) | 20 (7–44) | 0.41 |
| WCC (2/6) a | 10.9 (8.2–12.6) | 10.5 (9.0–11.9) | 0.95 |
| Platelets (1/8) a | 323 (236–412) | 293 (243–340) | 0.51 |
| Temperature (0/10) a | 38.6 (38.4–38.8) | 37.9 (37.4–38.1) | 0.0004 |
Number (per cent) or median with interquartile ranges.IL‐1Ra, IL‐6, TNF‐α, ICAM‐1 concentrations in picogram/litre, CRP in mg/l, WCC and platelets count 109/L, temperature in degrees Celsius.
Abbreviations: CRP, C‐reactive protein; ICAM, intracellular adhesion molecule; IL‐1Ra, interleukin‐1 receptor antagonist; IL‐6, Interleukin 6; TNF‐α, tumour necrosis factor alpha; WCC, white cell count.
Number of missing in the two groups, respectively.
4. DISCUSSION
The main result in this pilot study in aSAH patients was that lower GCS score, higher modified Fisher scale score and elevated serum concentrations of inflammatory markers (TNF‐α, IL‐6, IL‐1Ra, ICAM‐1, CRP) at admission were associated with unfavourable 1‐year outcome. Furthermore, infection, an elevated CRP and hydrocephalus 10 days after admission were also associated with unfavourable 1‐year outcome. After adjustments of the inflammatory markers were made, only the prevalence of an elevated level of IL1‐Ra on admission remained statistically significantly associated with unfavourable outcome.
The presence of infection and elevated temperature at day ten was significantly associated with development of DCI in an unadjusted analysis. The serum concentrations of inflammatory markers at admission were not significantly associated with the development of DCI in our cohort. Thus, in this study, the value of inflammatory markers (TNF‐α, IL‐6, IL‐1Ra, ICAM‐1, CRP), in predicting DCI and poor 1‐year outcome following aSAH when analysed from blood was limited. We could only demonstrate a significant association between inflammatory markers in sample 1 and unfavourable outcome in an unadjusted analysis. However, this may be the effect of a too small sample size, and a larger cohort may have yielded different results.
Inflammatory markers respond to many different clinical events that all affect the patient and the clinical course. If the primary haemorrhage is severe, the patient is at higher risk of non‐neurologic medical complications such as respiratory failure, cardiovascular complications, SIRS and infections. 1 , 35 , 36 An elevated level of inflammatory markers early in the clinical course may reflect a higher vulnerability or a higher risk for further complications as pointed out by Chamling et al. 37
We observed a correlation between an early elevation of IL‐1Ra and a poor 1‐year outcome. In 1997, Mathisen et al. 21 reported higher IL‐1Ra concentrations in patients with a poor clinical condition on day 12, as well as in patients who had an unfavourable outcome on day 3–10, suggesting that this marker might reflect the severity of the initial haemorrhage. A higher Fisher grade on admission was associated with poor 1‐year outcome in our study. A more substantial haemorrhage likely induces a more pronounced release of proinflammatory cytokines such as IL‐1. IL‐1, in turn, induces the release of other proinflammatory cytokines, for example, IL‐6, but it also induces the release of IL‐1Ra that counteracts and attenuates the effects of IL‐1. This could be an explanation for the high levels of IL‐1Ra and its association with poor outcome in our study. In fact, several recent studies indicate that IL‐1Ra may have protective effects in aSAH. 38 , 39 Still, we did not observe any association of IL‐1Ra with the development of DCI.
In our study, we demonstrated that elevated serum TNF‐α concentration early in the course indicated a poor outcome 1 year after admission in an unadjusted analysis, which is consistent with the results reported by Chou et al. in 2012 15 but contrasted with those reported by Rasmussen et al. in 2019. 26 In both previous studies, the outcome had been measured at 3 or 6 months after admission. Consistent with a 2011 report by Beeftink et al., 17 we did not observe a correlation between TNF‐α and DCI in our cohort. Moreover, our observation of an association between elevated serum IL‐6 concentration early in the course and a poor long‐term outcome after aSAH was consistent with the results of studies by Hölig et al. in 2015 16 and Muroi et al. in 2013, 24 in which the authors measured outcome after 3 and 6 months, respectively. Moreover, we found no significant association between IL‐6 and the development of DCI.
Furthermore, in our study an association between an elevated serum CRP concentration at admission and a poor outcome is in line with previous reports by Jeon et al. in 2012, 22 Srinivasan et al. in 2016 18 and Juvela et al., in 2012, 40 who measured outcome at discharge and after 3 months, respectively. Rasmussen et al. in 2019 26 found that high levels of plasma high sensitivity C‐reactive protein correlated to clinical outcome, and Csajbok et al. showed in 2015 41 that patients with a more significant rise in CRP had worse neurological outcome after 1 year. Jeon et al. 22 and Rothoerl et al. 42 observed that persistently elevated CRP concentrations at day 10 post‐aSAH was associated with DCI. This is consistent with our findings, although we did not achieve a statistical significance. We note that in an intensive care setting, an elevated CRP concentration after 10 days might be attributable to other factors, such as surgical procedures and infections. For example, 10 of 16 patients in the DCI group in our study had developed infections that could explain the persistent CRP levels.
Our observation between an early elevated serum ICAM‐1 concentration and a poor 1‐year outcome was consistent with the findings reported by Mack and Mocco in 2002. 43 However, those authors measured outcome after only 14 days. Consistent with Rasmussen et al. 26 and Chamling et al. in 2017, 37 we found no convincing association between ICAM‐1 and the development of DCI.
Increasing evidence suggests that inflammation contributes to both poor patient outcome and the development of DCI after aSAH. However, analyses of serum concentrations of inflammatory markers after aSAH are difficult, as the secretion of these factors is time‐dependent in relation to initial haemorrhage. This might be one possible explanation for the variable results in the studies mentioned above. Comparisons of existing studies are complicated because of differences in protocols concerning the types of inflammatory markers, the timing of sample collection and the outcomes of interest, as well as differences in the definitions of these outcomes. What type of fluid sample to collect and analyse in relation to clinical relevance is still somewhat unclear.
Levels of inflammatory markers are affected by infection, a common complication observed in patients with aSAH. These problems have recently been addressed by other authors such as Chou et al. in a 2019 report. 23 We suggest that future studies should focus on biomarkers sampled from blood and cerebrospinal fluid simultaneously at fixed time points after ictus so that possible spill over in patients with affected blood–brain barrier could be detected. Future studies need to compare better‐defined outcome at more fixed time points after ictus to increase comparability between studies.
In addition to the challenges mentioned above, this study had some limitations of note. This is a single‐centre study with a relatively small number of included patients. Inflammatory markers were measured only in serum samples and not concurrently in cerebrospinal fluid (CSF). Furthermore, our definition of unfavourable and favourable outcome related to the dichotomization of patients with GOSE 1–4 and 5–8 may also have affected the results.
Despite these limitations, our study included a patient cohort that is representative for the aSAH population in general. Results are consistent with those of several other studies that demonstrated an association between the early inflammatory response after aSAH and unfavourable outcome in an unadjusted analysis. In future studies larger cohorts and analyses of simultaneously collected CSF are warranted.
5. CONCLUSION
In this pilot study in aSAH patients, we found that elevated serum concentrations of inflammatory markers (TNF‐α, IL‐6, IL‐1Ra, ICAM‐1, CRP) sampled at admission were associated with a poor neurological outcome 1 year later. However, inflammatory markers are affected by many plausible clinical events, and when adjusted for clinical presentation on admission, only IL‐1 Ra remained statistically significantly associated with poor outcome. The robustness of these results needs to be tested in a larger trial.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Sandra Bjerkne Wenneberg participated in study planning, data analysis and manuscript writing. Helena Odenstedt Hergès participated in study planning, data analysis and manuscript writing. Pernilla Svedin performed blood sample analyses and participated in manuscript writing. Carina Mallard performed blood sample analyses and participated in manuscript writing. Thomas Karlsson performed statistical analysis and participated in manuscript writing and responses to reviewers. Martin Adiels performed statistical analysis and participated in manuscript writing. Silvana Naredi participated in study planning, data analysis and manuscript writing. Linda Block participated in study planning, data analysis, manuscript writing and project coordination.
Supporting information
ACKNOWLEDGEMENT
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐772521 and ALFGBG‐720511, ALFGBG‐74160) and the Healthcare Board, Region Västra Götaland (VGFOUREG‐753851, VGFOUREG‐833561 and VGFOUREG‐650891, VGFOUREG‐856851). The authors declare no conflict of interests.
Funding informationThis study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐772521 and ALFGBG‐720511, ALFGBG‐74160) and the Healthcare Board, Region Västra Götaland (VGFOUREG‐833561 and VGFOUREG‐856851).
REFERENCES
- 1. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655–666. [DOI] [PubMed] [Google Scholar]
- 2. Geraghty JR, Testai FD. Delayed cerebral ischemia after subarachnoid hemorrhage: beyond vasospasm and towards a multifactorial pathophysiology. Curr Atheroscler Rep. 2017;19(12):50. [DOI] [PubMed] [Google Scholar]
- 3. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–2395. [DOI] [PubMed] [Google Scholar]
- 4. Jaja BN, Cusimano MD, Etminan N, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: a systematic review. Neurocrit Care. 2013;18(1):143–153. [DOI] [PubMed] [Google Scholar]
- 5. Zhao B, Yang H, Zheng K, et al. Preoperative and postoperative predictors of long‐term outcome after endovascular treatment of poor‐grade aneurysmal subarachnoid hemorrhage. J Neurosurg. 2017;126(6):1764–1771. [DOI] [PubMed] [Google Scholar]
- 6. Beneš VR, Jurák L, Brabec R, et al. Causes of poor outcome in patients admitted with good‐grade subarachnoid haemorrhage. Acta Neurochir (Wien). 2017;159(3):559–565. [DOI] [PubMed] [Google Scholar]
- 7. Dasenbrock HH, Rudy RF, Smith TR, et al. Hospital‐acquired infections after aneurysmal subarachnoid hemorrhage: a nationwide analysis. World Neurosurg. 2016;88:459–474. [DOI] [PubMed] [Google Scholar]
- 8. Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10(1):44–58. [DOI] [PubMed] [Google Scholar]
- 9. Thelin EP, Tajsic T, Zeiler FA, et al. Monitoring the neuroinflammatory response following acute brain injury. Front Neurol. 2017;8:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S, Feng H, Sherchan P, et al. Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurogibol. 2014;115:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watson E, Ding D, Khattar NK, Everhart DE, James RF. Neurocognitive outcomes after aneurysmal subarachnoid hemorrhage: Identifying inflammatory biomarkers. J Neurol Sci. 2018;394:84–93. [DOI] [PubMed] [Google Scholar]
- 12. Stein SC, Browne KD, Chen XH, Smith DH, Graham DI. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery. 2006;59(4):781–788; discussion 787–788. [DOI] [PubMed] [Google Scholar]
- 13. Dreier JP, Major S, Manning A, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(Pt 7):1866–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahn SH, Savarraj JPJ, Parsha K, et al. Inflammation in delayed ischemia and functional outcomes after subarachnoid hemorrhage. J Neuroinflammation. 2019;16(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chou SH, Feske SK, Atherton J, et al. Early elevation of serum tumor necrosis factor‐alpha is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012;60(7):1054–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hollig A, Remmel D, Stoffel‐Wagner B, Schubert GA, Coburn M, Clusmann H. Association of early inflammatory parameters after subarachnoid hemorrhage with functional outcome: a prospective cohort study. Clin Neurol Neurosurg. 2015;138:177–183. [DOI] [PubMed] [Google Scholar]
- 17. Beeftink MM, Ruigrok YM, Rinkel GJ, van den Bergh WM. Relation of serum TNF‐alpha and TNF‐alpha genotype with delayed cerebral ischemia and outcome in subarachnoid hemorrhage. Neurocrit Care. 2011;15(3):405–409. [DOI] [PubMed] [Google Scholar]
- 18. Srinivasan A, Aggarwal A, Gaudihalli S, et al. Impact of early leukocytosis and elevated high‐sensitivity C‐reactive protein on delayed cerebral ischemia and neurologic outcome after subarachnoid hemorrhage. World Neurosurg. 2016;90:91–95. [DOI] [PubMed] [Google Scholar]
- 19. Frontera JA, Aledort L, Gordon E, et al. Early platelet activation, inflammation and acute brain injury after a subarachnoid hemorrhage: a pilot study. J Thromb Haemost. 2012;10(4):711–713. [DOI] [PubMed] [Google Scholar]
- 20. Rackauskaite D, Svanborg E, Andersson E, Lowhagen K, Csajbok L, Nellgard B. Prospective study: long‐term outcome at 12–15 years after aneurysmal subarachnoid hemorrhage. Acta Neurol Scand. 2018;138(5):400–407. [DOI] [PubMed] [Google Scholar]
- 21. Mathiesen T, Edner G, Ulfarsson E, Andersson B. Cerebrospinal fluid interleukin‐1 receptor antagonist and tumor necrosis factor‐alpha following subarachnoid hemorrhage. J Neurosurg. 1997;87(2):215–220. [DOI] [PubMed] [Google Scholar]
- 22. Jeon YT, Lee JH, Lee H, et al. The postoperative C‐reactive protein level can be a useful prognostic factor for poor outcome and symptomatic vasospasm in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2012;24(4):317–324. [DOI] [PubMed] [Google Scholar]
- 23. Chou SH, Macdonald RL, Keller E. Biospecimens and molecular and cellular biomarkers in aneurysmal subarachnoid hemorrhage studies: common data elements and standard reporting recommendations. Neurocrit Care. 2019;30(Suppl 1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muroi C, Hugelshofer M, Seule M, et al. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72(3):367–375; discussion 375. [DOI] [PubMed] [Google Scholar]
- 25. Wu W, Guan Y, Zhao G, et al. Elevated IL‐6 and TNF‐alpha Levels in cerebrospinal fluid of subarachnoid hemorrhage patients. Mol Neurobiol. 2016;53(5):3277–3285. [DOI] [PubMed] [Google Scholar]
- 26. Rasmussen R, Bache S, Stavngaard T, Moller K. Plasma levels of IL‐6, IL‐8, IL‐10, ICAM‐1, VCAM‐1, IFNgamma, and TNFalpha are not associated with delayed cerebral ischemia, cerebral vasospasm, or clinical outcome in patients with subarachnoid hemorrhage. World Neurosurg. 2019;128:e1131‐e1136. [DOI] [PubMed] [Google Scholar]
- 27. Jabbarli R, Pierscianek D, Darkwah Oppong M, et al. Laboratory biomarkers of delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review. Neurosurg Rev. 2018;43(3):825–833. [DOI] [PubMed] [Google Scholar]
- 28. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. [DOI] [PubMed] [Google Scholar]
- 29. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2012;43(6):1711–1737. [DOI] [PubMed] [Google Scholar]
- 30. Report of world federation of neurological surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68(6):985–986. [DOI] [PubMed] [Google Scholar]
- 31. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. [DOI] [PubMed] [Google Scholar]
- 32. Frontera JA, Claassen J, Schmidt JM, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21–27; discussion 21–27. [DOI] [PubMed] [Google Scholar]
- 33. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. [DOI] [PubMed] [Google Scholar]
- 34. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 35. Chen S, Li Q, Wu H, Krafft PR, Wang Z, Zhang JH. The harmful effects of subarachnoid hemorrhage on extracerebral organs. Biomed Res Int. 2014;2014:858496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gruber A, Reinprecht A, Illievich UM, et al. Extracerebral organ dysfunction and neurologic outcome after aneurysmal subarachnoid hemorrhage. Crit Care Med. 1999;27(3):505–514. [DOI] [PubMed] [Google Scholar]
- 37. Chamling B, Gross S, Stoffel‐Wagner B, et al. Early diagnosis of delayed cerebral ischemia: possible relevance for inflammatory biomarkers in routine clinical practice? World Neurosurg. 2017;104:152–157. [DOI] [PubMed] [Google Scholar]
- 38. Singh N, Hopkins SJ, Hulme S, et al. The effect of intravenous interleukin‐1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J Neuroinflammation. 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Galea J, Ogungbenro K, Hulme S, et al. Reduction of inflammation after administration of interleukin‐1 receptor antagonist following aneurysmal subarachnoid hemorrhage: results of the subcutaneous interleukin‐1Ra in SAH (SCIL‐SAH) study. J Neurosurg. 2018;128(2):515–523. [DOI] [PubMed] [Google Scholar]
- 40. Juvela S, Kuhmonen J, Siironen J. C‐reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien). 2012;154(3):397–404. [DOI] [PubMed] [Google Scholar]
- 41. Csajbok LZ, Nylen K, Ost M, Sonander H, Nellgard B. In‐hospital C‐reactive protein predicts outcome after aneurysmal subarachnoid haemorrhage treated by endovascular coiling. Acta Anaesthesiol Scand. 2015;59(2):255–264. [DOI] [PubMed] [Google Scholar]
- 42. Rothoerl RD, Axmann C, Pina AL, Woertgen C, Brawanski A. Possible role of the C‐reactive protein and white blood cell count in the pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol. 2006;18(1):68–72. [DOI] [PubMed] [Google Scholar]
- 43. Mack WJ, Mocco J, Hoh DJ, et al. Outcome prediction with serum intercellular adhesion molecule‐1 levels after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;96(1):71–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


