Figure 2: Microbiota-mediated regulation of anti-cancer therapies and immune therapy.
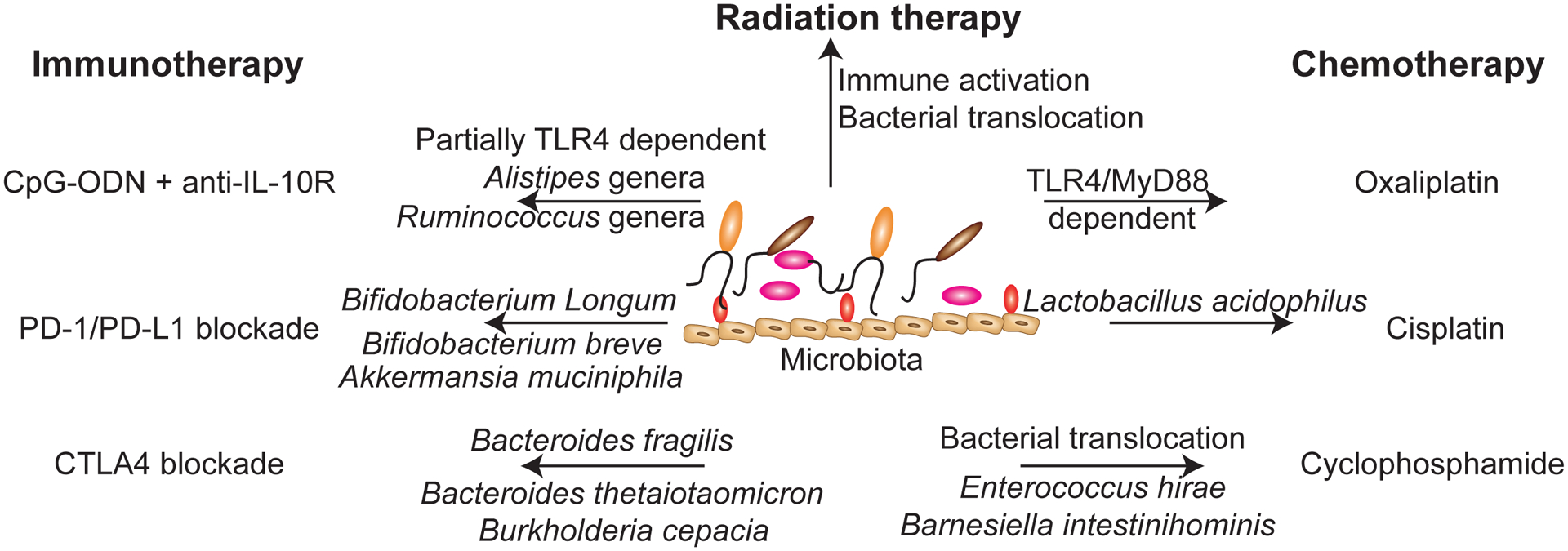
Recent human and mouse studies have identified commensal microorganisms or microbiota-dependent mechanisms that enhance the efficacy of immune therapies, radiation therapy, and chemotherapy. Systemic delivery of the TLR9 agonist, CpG Oligodeoxynucleotide (ODN) in combination with IL-10 blockade is partially TLR4 dependent. Alistipes and Ruminococcus genera were found to be overrepresented in treatment-responsive mice, whereas mono-colonization with A. shahii was sufficient to restore response to this therapy in mice that were treated with antibiotics. For PD-1/PD-L1 blockade, studies have demonstrated that mono-colonization with Bifidobacterium longum, B. breve, and Akkermansia muciniphila were sufficient to enhance the efficacy of this therapy in tumor-bearing mice. For blockade of CTLA4 signaling, gavage of antibiotic-treated mice with Bacteroides fragilis, B. thetaiotamicron, and Burkholderia cepacian were sufficient to restore efficacy of this therapy in antibiotic-treated or germ-free mice. Radiation therapy on the other hand can alter microbiome composition and associate with gut toxicity, commensal translocation, and pathologic inflammation. However, total body irradiation is sufficient to improve the efficacy of adoptive T cell transfer due to the translocation of gram-negative species into the mesenteric lymph nodes and TLR4 signaling. For chemotherapies, oxaliplatin-induced cell death is dependent upon TLR4 signaling whereas the anti-tumor responses are MyD88 dependent. Recolonization of antibiotic-treated mice with Lactobacillus acidophilus is sufficient to restore the efficacy of cisplatin in tumor-bearing antibiotic-treated mice. The beneficial effects of cyclophosphamide require the translocation of gram-positive bacteria from the gut into secondary lymphoid organs, where commensals such as Enterococcus hirae act to prime T cells in a MyD88-dependent manner. Additionally, gram-negative Barnesiella intestinihominis associates with enhanced cyclophosphamide-mediated anti-tumor immunity. Mono-colonization with either microorganism is able to partially restore the immunogenic effects of cyclophosphamide in antibiotic-treated mice.
