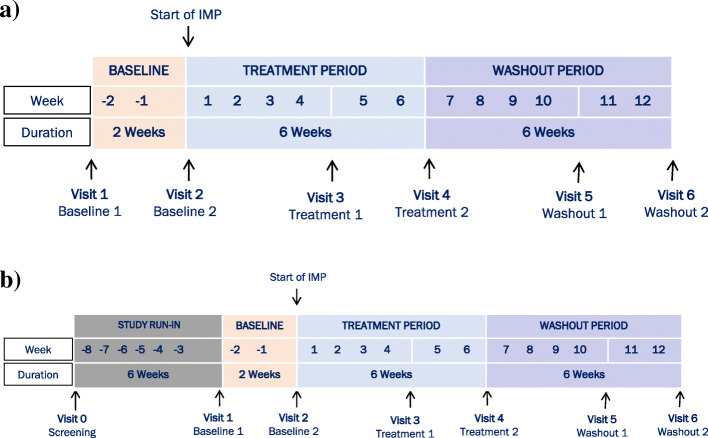
Fig. 1.
Parent study schema. During the treatment period, all patients will receive N-acetyl-l-leucine for 42 days (+ 7 days). Visit 3 (treatment 1) will occur at day 28 (+ 7 days) of the treatment period and visit 4 (treatment 2) will occur after the full 42 days (+ 7 days) of treatment. A 42-day (+ 7 days) washout period will be performed following treatment with N-acetyl-l-leucine. Visit 5 (washout 1) will occur on day 28 (+ 7 days) of the washout period and visit 6 (washout 2) will occur after the full 42 days (+ 7 days) of washout. a Naïve patients screening pathway: patients who have not used any prohibited medications within 42 days of screening are “naïve.” Their initial screening visit is treated as visit 1 (baseline 1). b Non-naïve patient screening pathway: patients who have used or are unable to confirm or deny if they have used any prohibited medication within the past 42 days are “non-naïve.” Patient will be given the opportunity to undergo a minimum of 42 days washout before returning for a repeat visit 1 (baseline 1)