Figure 4.
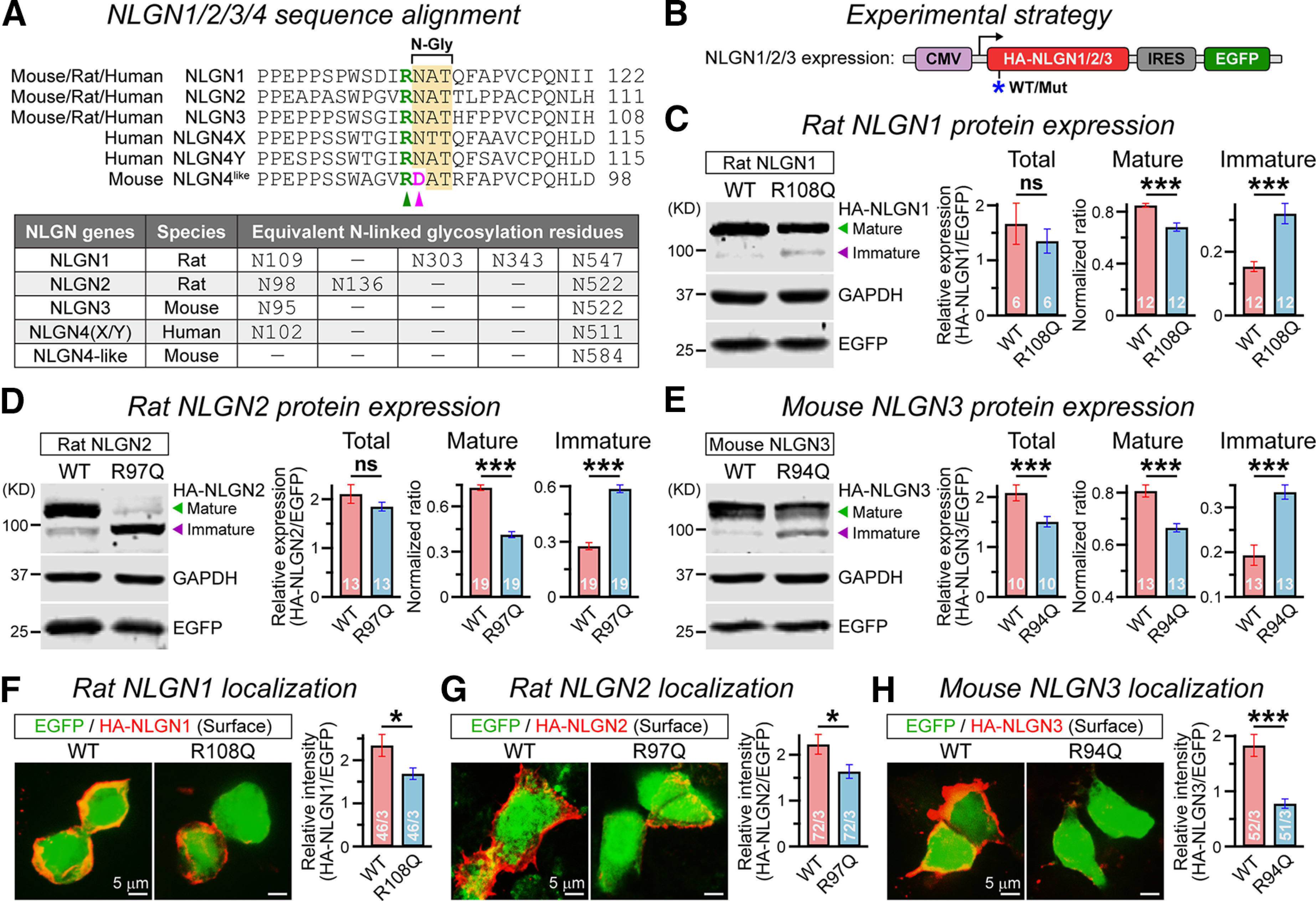
R101Q-equivalent mutations similarly affect maturation and surface trafficking of all NLGNs. A, Sequence alignment (top) of mouse/rat/human versions of NLGN1/2/3/4×/4Y indicate a conserved arginine residue (green arrowhead) followed by an asparagine glycosylation site (N-Gly) in all NLGNs, except in mouse NLGN4 that contains an aspartate residue instead (pink arrowhead). No NLGN4 homolog was identified in the rat. The table (bottom) lists all comparable N-linked glycosylation sites of NLGN1/2/3/4 from different species. B, Strategy for expressing HA-tagged NLGN1/2/3 with or without the R101Q-equivalent mutations (for details, see Materials and Methods). C, Representative immunoblot (left) and normalized ratios (right) of HA-tagged mature (green arrowhead) versus immature (purple arrowhead) NLGN1 products extracted from HEK293 cells expressing rat NLGN1 WT or R108Q mutant proteins. EGFP and GAPDH were respectively used as transfection control and loading control. D, E, Same as C, except for rat NLGN2 containing R97Q mutation (D), and mouse NLGN3 containing R94Q mutation (E). F–H, Sample images (left) represent immunostaining of HA-tagged NLGNs under nonpermeabilized conditions in HEK293 cells coexpressing IRES-driven EGFP, and summary graphs (right) of HA-NLGN signal intensity relative to EGFP, for rat NLGN1 WT versus R108Q mutant (F), rat NLGN2 WT versus R97Q mutant (G), and mouse NLGN3 WT versus R94Q mutant (H). All summary data are presented as the mean ± SEM, with the total number of independent replicates (C–E) or fields of view (F–H)/number of experimental batches. Statistical significance was assessed by two-tailed, paired (C–E) or unpaired (F–H), Student's t test. ***p < 0.005; *p < 0.05; ns, not significant (p > 0.05).
