Figure 6.
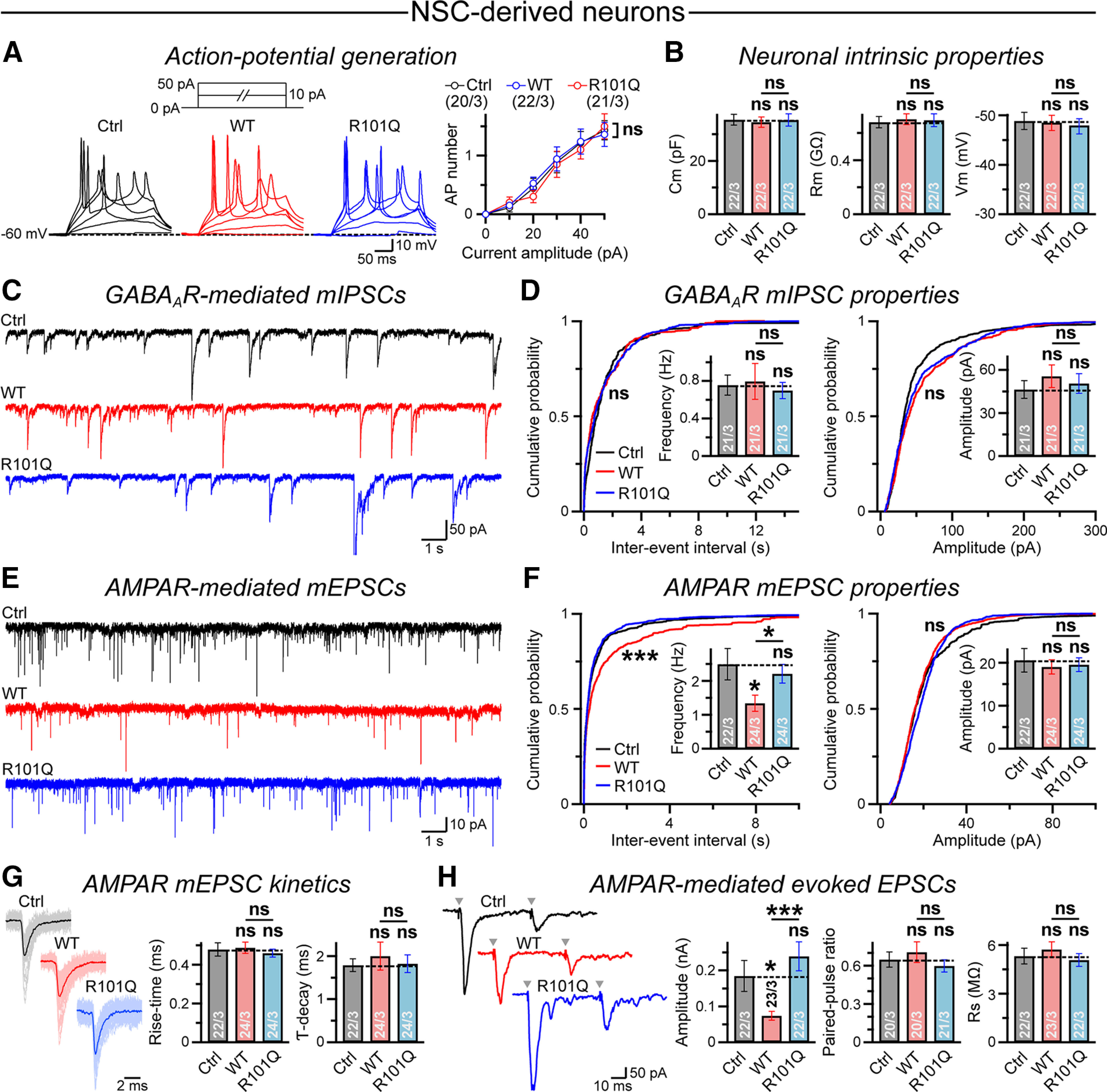
NLGN4 WT but not the R101Q variant suppresses AMPAR-mediated synaptic transmission in NSC-derived human neurons. A, Recordings were conducted from NSC-derived neurons expressing either a control vector (Ctrl; EGFP only) or NLGN4 WT versus R101Q mutant constructs followed by an IRES-driven EGFP reporter. Sample traces (left) of APs induced by step current injections (protocol is shown on top, Vhold = −60 mV), and summary plot (right) of AP numbers as a function of injected current–amplitude. B, Average values of Cm (left), Rm (middle), and Vm (right), as measured from control, NLGN4 WT, and R101Q conditions. C, D, Example traces of GABAAR-mediated mIPSCs (C) and cumulative plots with average bar graphs (D) for mIPSC event frequency and amplitude, as recorded from control, NLGN4 WT, and R101Q conditions. E, F, Same as C and D, except for AMPAR-mediated mEPSC events. G, Superimposed mEPSC waveforms (left) from control, NLGN4 WT, and R101Q conditions. Summary graphs (right) of mEPSC rise time and decay kinetics. H, Representative traces (left) of AMPAR-mediated evoked EPSCs stimulated in pairs (Δt = 50 ms); arrowheads indicate stimulation artifacts. Summary graphs (right) of EPSC amplitude, PPR (EPSC2/EPSC1) as an indirect measure of presynaptic release probability, and Rs as a measure of recording quality. All quantifications are the mean ± SEM. All summary data include the number of cells patched/experimental batches. Statistical significance was calculated by two-tailed, unpaired, Student's t test (all bar graphs), one-way ANOVA (summary plot; A), or KS test (WT vs R101Q probability plots; D–F). ***p < 0.005; *p < 0.05; ns, not significant (p > 0.05).
