ABSTRACT
Background:
The diagnostic value and suitability of prostate cancer antigen 3 (PCA3) for the detection of prostate cancer (PCa) have been inconsistent in previous studies. Thus, the aim of the present meta-analysis was performed to systematically evaluate the diagnostic value of PCA3 for PCa.
Materials and Methods:
A meta-analysis was performed to search relevant studies using online databases EMBASE, PubMed and Web of Science published until February 1st, 2019. Ultimately, 65 studies met the inclusion criteria for this meta-analysis with 8.139 cases and 14.116 controls. The sensitivity, specificity, positive likelihood ratios (LR+), negative likelihood ratios (LR−), and other measures of PCA3 were pooled and determined to evaluate the diagnostic rate of PCa by the random-effect model.
Results:
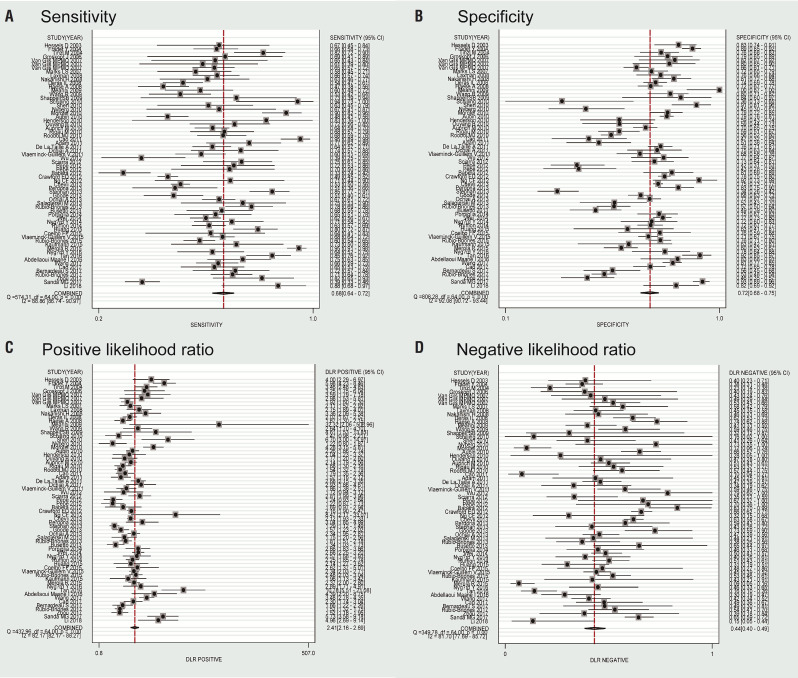
With PCA3, the pooled overall diagnostic sensitivity, specificity, LR+, LR−, and 95% confidence intervals (CIs) for predicting significant PCa were 0.68 (0.64-0.72), 0.72 (0.68-0.75), 2.41 (2.16-2.69), 0.44 (0.40-0.49), respectively. Besides, the summary diagnostic odds ratio (DOR) and 95% CIs for PCA3 was 5.44 (4.53-6.53). In addition, the area under summary receiver operating characteristic (sROC) curves and 95% CIs was 0.76 (0.72-0.79). The major design deficiencies of included studies were differential verification bias, and a lack of clear inclusion and exclusion criteria.
Conclusions:
The results of this meta-analysis suggested that PCA3 was a non-invasive method with the acceptable sensitivity and specificity in the diagnosis of PCa, to distinguish between patients and healthy individuals. To validate the potential applicability of PCA3 in the diagnosis of PCa, more rigorous studies were needed to confirm these conclusions.
Keywords: prostate cancer antigen 3, human [Supplementary Concept]; Prostate cancer, familial [Supplementary Concept]; Meta-Analysis [Publication Type]
INTRODUCTION
Prostate cancer (PCa) is a worldwide diagnosed malignant neoplasm, which has become the second mortality rate of tumors in elderly men (1-3). The clinic symptoms of PCa are mostly similar to benign prostatic hyperplasia (BPH), which makes a difficulty for clinician to accurately distinguish PCa from BPH (4). Due to lack of effective and timely diagnostic methods, the prognosis of PCa was generally poor (4). It is quiet important for clinicians to the detection of PCa at an early stage, in order to reduce the mortality of PCa, improve the survival rate and increase the opportunity of effective medical interventions (5-7).
Nowadays, serum prostate-specific antigen (PSA) is still widely used for PCa screening (5, 8). Serum PSA level has been widely used to detect PCa, which is an organ-specific antigen, but not a cancer-specific antigen (9). Several diseases, including BPH, prostatitis and PCa, might be associated with an elevated PSA level (5, 9). Though a high level of PSA is likely to be associated with PCa, the low specificity of PSA limits its use as a screening test and unnecessary biopsies (10). As a noninvasive diagnostic urine test, prostate cancer gene 3 (PCA3) is more accurate than PSA and can reduce the likelihood of false-positive results (11). Up to present, numerous individual studies have been performed to explore the diagnostic value of urine PCA3 in the management of PCa (12-18). However, these studies on the diagnostic performance of PCA3 have reported unclear or even conflicting results.
Based on a systematic review with meta—analysis, the objective of this study was to systematically collect the databases search results and perform an updated meta-analysis to assess the efficacy of diagnostic tests of PCA3 for the early detection of PCa.
MATERIALS AND METHODS
Literature search strategy
Studies were searched in the electronic databases EMBASE, PubMed and Web of Science up to February 1st, 2019. Available publications were identified using the following keywords or text words: ‘Differential Display clone 3’ or ‘DD3’ or ‘prostate cancer antigen 3’ or ‘PCA3’, ‘prostate cancer’ or ‘prostate neoplasms’ or ‘prostate carcinoma’ or ‘prostatic cancer’ or ‘prostatic neoplasm’ or ‘prostatic carcinoma’ or ‘cancer of prostate’ or ‘neoplasms of prostate’ or ‘carcinoma of prostate’, and ‘sensitivity’ or ‘specificity’ or ‘false negative’ or ‘false positive’ or ‘diagnosis’ or ‘detection’ or ‘accuracy’. For assessing all relevant studies, the most eligible literatures were retrieved. Moreover, relevant articles from reference lists of selected articles were searched to identify more relevant publications and avoid relevant information missing. No language restriction was applied.
There is no registered protocol for this systematic review. This systematic review and meta-analysis was conducted in accordance with the PRISMA guidelines, which compile guidelines for the reporting of meta-analysis of observational studies. The relevant studies included in this meta-analysis are previously published, and therefore, ethical approval and informed consent are not required.
Criteria for inclusion and exclusion of published studies
The included studies must meet the inclusion criteria: (1) A case-control, nested case-control, or cohort randomized prospective or retrospective study, (2) Evaluate the diagnostic value of PCA3 in patients with PCa, (3) Available data for extraction to calculate sensitivity, specificity and other measures, (4) When duplications or the same patients used in several publications existed, the most recent or complete study was chosen in this meta-analysis. Additionally, the major exclusion criteria were as follows: (1) No available data; (2) Non-case-control studies, case reports, letters, reviewed editorial articles, (3) Duplicated publications with previous studies.
Data extraction
The extracted appropriate information and data with a standard protocol were inspected by two researchers independently, to ensure the reliability and accuracy of the results. Moreover, the controversies were reviewed and settled through discussion by a third investigator, until all problems were finally resolved. The following information from each study were extracted: name of first author, publication date, country, ethnicity, mean age, PSA value (ng/mL), assay type, sample source, sample size, cut-off value, controls value (ng/ mL), PCa/non-PCa case, and raw data including true positive (TP), true negative (TN), false positive (FP), and false negative (FN) results.
In addition, the quality of each reference was also evaluated by two investigators independently, according to the revised QUADAS tools (19). Each domain contains seven questions, which can be answered by “yes”, “no” or “not clear” that assess the quality of included studies. An answer of “yes” means a low risk of bias, whereas “no” or “not clear” means a higher risk of bias in terms of the loss of some information from each literature.
Statistical analysis
The statistical software STATA version 12.0 (StataCorp LP, College Station, TX) was performed to conduct all statistical data in this meta-analysis, and the Spearman test was used to analyze the threshold effect or the non-threshold effect. All of the statistical tests were two-sided, and P <0.05 was considered statistically significant. The pooled sensitivity, specificity, positive likelihood ratios (LR+), negative likelihood ratios (LR−), and the diagnostic odds ratio (DOR) as well as their corresponding 95% CIs were summarized to assess the diagnostic value of PCA3 in patients with PCa. Data were visualized as forest plots and receiver operating characteristic curves (ROC). The between-study heterogeneity was evaluated by Q test and I2 statistic, and P <0.05 was deemed statistically significant. As a quantitative measurement of inconsistency across different studies, I2-square value, ranged from 0 (no observed heterogeneity) to 100% (maximal heterogeneity), was also calculated. If the heterogeneity across studies was not identified, the fixed-effects model was used. Otherwise, the random-effects model was used in the meta-analysis. In addition, the summary receiver operating characteristic (sROC) curve was generated and the area under sROC curves (AUC) was calculated both overall and the subgroup analysis. Additionally, publication bias was investigated using Deek's funnel plot asymmetry test. When the P value of the Egger test was <0.05, the statistical significance was defined. Then, we replicated the funnel plot with its “missing” counterparts around the adjusted summary estimate.
RESULTS
Studies characteristics
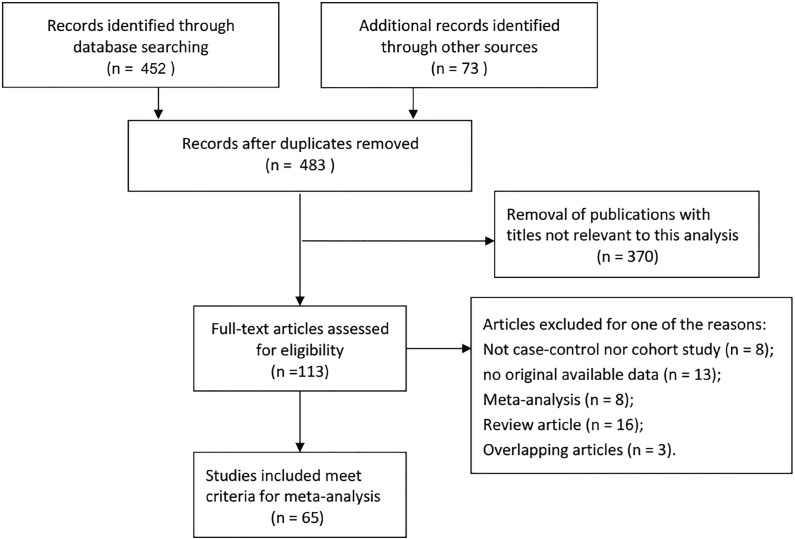
As shown in Figure-1, 483 records were retrieved. After screening titles and abstracts of relevant articles, 418 articles were excluded because these were not related to the inclusion criteria. Finally, 65 case-control studies published between 2003 and 2018 were included in the meta-analysis (11-18, 20-76). All of these studies were retrospective in design.
Figure 1. Flowchart of literature search and selection process.
The present meta-analysis included 8.139 cases and 14.116 controls from a total of 65 case-control studies about evaluating the diagnostic value of PCA3 in patients with PCa, and the detailed data of each study are listed in Table-1. Based on the studies described above, we retrieved data from 22.255 patients with PCA3 test and 5.065 patients with diagnosed PCa. All the studies presented the sensitivity, specificity, LR+, LR− and cut-off points. In these studies, these assay types, such as enzyme-linked immunosorbent assay (ELISA) and reverse transcription-polymerase chain reaction (RT-PCR), were applied to detect the expression level of PCA3. Besides, fifty studies were performed on Caucasian population, ten studies were conducted on Asian population, one study was carried out on African population, and the remaining studies involved more than one race.
Table 1. Characteristics and methodology assessment of individual studies included in the meta-analysis.
| Year | First author | Country | Ethnicity | T Mean age (years) | T Mean PSA (ng/mL) | Assay type | Sample source | Cut-off value | Case | Control | TP | FP | FN | TN | QUADA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | Li | China/Asian | Asian | NR | NR | PCR | Urine | 33.9 | 24 | 53 | 21 | 9 | 3 | 42 | 12 |
| 2017 | Sanda MG | US | Caucasian/Asian/African | 62 (33-85) | 4.8 *(0.3-460.4) | PCR | Urine | 20 | 264 | 262 | 104 | 18 | 160 | 244 | 10 |
| 2017 | Zhou | China/Asian | Asian | 65.3±7.8 | 7.1±1.77 | PCR | Urine | 23.5 | 33 | 89 | 27 | 48 | 6 | 41 | 11 |
| 2017 | Rubio-Briones | Spain | Caucasian | 61.7±6.12 | 4.49±1.99 | PCR | Urine | 35 | 161 | 396 | 115 | 186 | 46 | 210 | 11 |
| 2017 | Bernardeau S | France | Caucasian | 66.5 | 5.6 | PCR | Urine | 24 | 47 | 78 | 34 | 34 | 13 | 44 | 10 |
| 2017 | Cao | US | Caucasian/African | 63* (59–68) | NR | PCR | Urine | 35 | 77 | 195 | 50 | 55 | 27 | 140 | 12 |
| 2017 | Wang | China/Asian | Asian | 45-92 | NR | PCR | Urine | 40.38 | 169 | 425 | 112 | 81 | 57 | 344 | 12 |
| 2016 | Abdellaoui Maane I | Morocco | Caucasian | 52-73 | 6.16-15.9 | PCR | Tissue | cutoff 1.035 | 64 | 41 | 48 | 7 | 16 | 34 | 11 |
| 2016 | Tan | China/Asian | Asian | 71 (60-89) | 32.4 (2.5-199.7) | LAMP | Serum | NR | 89 | 101 | 76 | 8 | 13 | 93 | 10 |
| 2016 | Nygård Y | Norway | Caucasian | 64.0 (65.1*; 62.9-65.2a) | 9.1 (7.2*;8.3-9.9a) | PCR | Urine | 35 | 70 | 54 | 45 | 12 | 25 | 42 | 10 |
| 2015 | Merola R | Italy | Caucasian | NR | NR | PCR | Urine | 51 | 195 | 212 | 185 | 85 | 10 | 127 | 11 |
| 2015 | Kaufmann | Germany | Caucasian | 65 ± 5.6 (52-79) | 10 ± 4.4 (4.0-25.0) | PCR | Urine | 35 | 22 | 27 | 16 | 10 | 6 | 17 | 12 |
| 2015 | Rubio-Briones | Spain | Caucasian | 64(58-69) | 5.2(4.3-7.2) | PCR | Urine | 35 | 318 | 374 | 190 | 90 | 128 | 284 | 11 |
| 2015 | Vlaeminck-Guillem V | France | Caucasian | 64 ± 7(64*,59-69) | 6.2 ± 4.3(6.6*,5-9.4) | PCR | Urine | 35 | 480 | 535 | 326 | 155 | 154 | 380 | 10 |
| 2015 | Coelho FF | Brasil | Caucasian | 65.8±7.35 | NR | PCR | Urine | cutoff 0.2219 | 22 | 37 | 14 | 9 | 8 | 28 | 12 |
| 2015 | Huang | China/Asian | Asian | 70*(51-88) | 13.67(7.98–29.02)b | PCR | Urine | 35 | 112 | 24 | 90 | 9 | 22 | 15 | 11 |
| 2014 | Ruffion | France | Caucasian | 63(58-67)b | 5.9(4.7-7.9)b | PCR | Urine | 35 | 274 | 321 | 173 | 90 | 101 | 231 | 11 |
| 2014 | Nygård Y | Norway | Caucasian | 54.0 ± 6.4; 65.1* | 9.1 ± 4.7; 7.2* | PCR | Urine | 35 | 59 | 65 | 42 | 18 | 17 | 47 | 10 |
| 2014 | Wei | US | Caucasian/Asian/African | 62±8 | 8±14 | PCR | Urine | 35 | 331 | 528 | 205 | 122 | 126 | 406 | 13 |
| 2014 | Porpiglia | Italy | Caucasian | 65 (60-70)b | 6.9 (5.2-9.8)b | PCR | Urine | 32.5 | 52 | 118 | 34 | 29 | 18 | 89 | 10 |
| 2014 | Chevli | US | Caucasian | 64.8± 9.2 | 6.4±23.3c | PCR | Urine | 35 | 902 | 2171 | 478 | 543 | 424 | 1628 | 12 |
| 2013 | Busetto | Italy/Rome | Caucasian | 66.4 ± 5.3 | 6.8± 1.6 | PCR | Urine | 35 | 68 | 95 | 46 | 48 | 22 | 47 | 11 |
| 2013 | Rubio-Briones | Spain | Caucasian | 57.5±6.2 (57*, 40-74) | 4.63±2.25 (4.04*, 0.37-19.5) | PCR | Urine | 35 | 105 | 216 | 82 | 93 | 23 | 123 | 10 |
| 2013 | Salagierski | Poland/Europe | Caucasian | 66.2±6.8 | 7.5±1.9 | PCR | Urine | 35 | 24 | 56 | 18 | 24 | 6 | 32 | 11 |
| 2013 | Ochiai | Japan | Asian | 69*(42–89) | 7.6 *(1.4–1908) | PCR | Urine | 35 | 264 | 369 | 176 | 105 | 88 | 264 | 11 |
| 2013 | Goode | US | Caucasian | 66*(41–90) | 4.8*(0.1–54.2) | PCR | Urine | 35 | 95 | 361 | 48 | 116 | 47 | 245 | 11 |
| 2013 | Stephan | Germany/Europe | Caucasian | 65 *(41–81) | 6.05 (0.50–19.77) | PCR | Urine | 28 | 110 | 136 | 94 | 90 | 16 | 46 | 12 |
| 2012 | Perdona | Italy/Europe | Caucasian | 64.91±7.37 | 6.13 *(4.46–7.93)b | PCR | Urine | 32.5 | 47 | 113 | 24 | 19 | 23 | 94 | 10 |
| 2012 | Ng CF | China/Asian | Asian | 71 (56-86) | 20/10* (2-127) | PCR | Urine | 35 | 17 | 24 | 12 | 2 | 5 | 22 | 12 |
| 2012 | Crawford | US | Caucasian | 64.4±8.6 | 8.0±20.0 | PCR | Urine | 35 | 802 | 1111 | 389 | 249 | 413 | 862 | 12 |
| 2012 | Babera | Italy/Europe | Caucasian | 64* | 9.5*(3.7-28) | PCR | Urine | 35 | 110 | 67 | 36 | 13 | 74 | 54 | 10 |
| 2012 | Pepe | Italy/Europe | Caucasian | 64*(48-74) | 8.9*(4.5-10) | PCR | Urine | 35 | 27 | 47 | 19 | 27 | 8 | 20 | 11 |
| 2012 | Pepe | Italy/Europe | Caucasian | 62.5*(48-72) | 8.5 * (3.7-24) | PCR | Urine | 35 | 32 | 86 | 23 | 50 | 9 | 36 | 11 |
| 2012 | Sciarra | Italy/Europe | Caucasian | 63.7±7.24 | 6.98±2.86 | PCR | Urine | 35 | 55 | 113 | 41 | 30 | 14 | 83 | 10 |
| 2012 | Wu | US | Caucasian | 63.5±7.4 | 11.0±8.5 | PCR | Urine | 35 | 46 | 57 | 18 | 13 | 28 | 44 | 11 |
| 2011 | Vlaeminck-Guillem V | France | Caucasian | 63 ± 7 | 6.2 ± 4.3 | PCR | Urine | 35 | 126 | 114 | 76 | 37 | 50 | 77 | 11 |
| 2011 | Ochiai | Japan | Asian | 66*(44-87) | 7.2*(3.3-720.6) | PCR | Urine | 35 | 35 | 67 | 26 | 17 | 9 | 50 | 11 |
| 2011 | De La Taille A | France/Germany/Europe | Caucasian | 63.0± 7.6 | 5.9 ± 2.1 | PCR | Urine | 35 | 207 | 309 | 133 | 74 | 74 | 235 | 12 |
| 2011 | Adam | South Africa | African | 67(35–89) | NR | PCR | Urine | 35 | 44 | 61 | 34 | 30 | 10 | 31 | 11 |
| 2010 | Cao | China/Asian | Asian | NR | NR | PCR | Urine | AUC:0.73 | 86 | 45 | 82 | 24 | 4 | 21 | 10 |
| 2010 | Roobol | Netherlands/Europe | Caucasian | 70.07(63.7–74.0) | 2.74 (0.2–23.0) | PCR | Urine | 35 | 122 | 599 | 83 | 265 | 39 | 334 | 11 |
| 2010 | Rigau | Spain/Europe | Caucasian | 65.7 (44–85) | 11.86 (1.5–189) | PCR | Urine | 35 | 73 | 142 | 50 | 58 | 23 | 84 | 12 |
| 2010 | Auprich | France/Germany/Europe | Caucasian | 63(35–90) | 7.3(1–82.7) | PCR | Urine | 35 | 255 | 366 | 164 | 110 | 91 | 256 | 12 |
| 2010 | Ouyang | US | Caucasian | NR | NR | PCR | Urine | 19 | 43 | 49 | 31 | 20 | 12 | 29 | 10 |
| 2010 | Henderson | England/The Netherlands | Caucasian | 69.9 | 10.1(3.03-44.2) | PCR | Urine | 35 | 6 | 44 | 5 | 18 | 1 | 26 | 11 |
| 2010 | Aubin | US | Caucasian | NR | (0.30-33.9) | PCR | Urine | 35 | 190 | 882 | 92 | 189 | 98 | 693 | 12 |
| 2010 | Morote | Spain | Caucasian | 64* (39–85) | 6.4*(1.5–189) | PCR | Urine | NR | 83 | 161 | 75 | 34 | 8 | 127 | 11 |
| 2010 | Nyberg | Sweden/Europe | Caucasian | 63 *(57–70)b | 7.9 *(5.1–12.8)b | PCR | Urine | 35 | 18 | 44 | 12 | 24 | 6 | 20 | 10 |
| 2010 | Shen | China/Asian | Asian | 70.3(51–86) | NR | PCR | Urine | cutoff 0.107 | 35 | 64 | 22 | 6 | 13 | 58 | 10 |
| 2010 | Schilling | Germany/Europe | Caucasian | NR | 7.7*(2.0–46.9) | ELISA | Urine | 35 | 18 | 14 | 17 | 9 | 1 | 5 | 10 |
| 2009 | Shappell | US | Caucasian | NR | NR | PCR | Urine | 35 | 11 | 19 | 8 | 3 | 3 | 16 | 11 |
| 2009 | Wang | US | Caucasian | 62 ±8.3(44-86) | 8.7±12.4 | PCR | Urine | 35 | 87 | 100 | 46 | 20 | 41 | 80 | 10 |
| 2009 | Mearini | Italy/Europe | Caucasian | 69.1(53–83) | 1.08-172.0 | PCR | Urine | AUC: 0.814 | 70 | 26 | 42 | 0 | 28 | 26 | 10 |
| 2008 | Haese | Europe | Caucasian | 64.4±6.6 | 8.9 ± 7.6 | PCR | Urine | 35 | 128 | 335 | 60 | 94 | 68 | 241 | 11 |
| 2008 | Deras | US/Canada | Caucasian | 64 (32–89) | 7.8 (0.3–484) | PCR | Urine | 35 | 206 | 357 | 111 | 93 | 95 | 264 | 12 |
| 2008 | Nakanishi | US | Caucasian/African | 60 (45–70) | 5.7 (1.0–27.0) | PCR | Urine | 25 | 40 | 102 | 25 | 19 | 15 | 83 | 12 |
| 2008 | Laxman | US | Caucasian | NR | NR | PCR | Urine | AUC:0.66 | 138 | 96 | 91 | 23 | 47 | 73 | 11 |
| 2007 | Marks | US/Canada | Caucasian | 64 ± 7(64*45-83) | 7.4 ± 4.3(6.1*2.5-31.1) | PCR | Urine | 35 | 60 | 166 | 35 | 46 | 25 | 120 | 11 |
| 2007 | Van Gils MPMQ | Netherlands/Europe | Caucasian | 64.3±7.2 | 7.49 ± 2.93 | PCR | Urine | 58 | 174 | 360 | 113 | 122 | 61 | 238 | 12 |
| 2007 | Van Gils MPMQ | Netherlands/Europe | Caucasian | 64±7.2 | 8.73± 6.61 | PCR | Urine | 43 | 23 | 44 | 14 | 9 | 9 | 35 | 10 |
| 2007 | Van Gils MPMQ | Netherlands/Europe | Caucasian | NR | NR | PCR | Urine | 66 | 23 | 44 | 15 | 8 | 8 | 36 | 10 |
| 2006 | Groskopf | US | Caucasian | 67±11 (45-93) | 7.7±14.1(0.4-101.7) | PCR | Urine | cutoff 0.05 | 16 | 52 | 11 | 11 | 5 | 41 | 12 |
| 2004 | Tinzl | Austria/Europe | Caucasian | 64.7 (41-89) | 0.59 -1486 | PCR | Urine | cutoff 0.5 | 79 | 122 | 65 | 29 | 14 | 93 | 13 |
| 2004 | Fradet | Canada | Caucasian | 64* (40-87) | 0.1-144 | PCR | Urine | cutoff 0.5 | 152 | 291 | 100 | 32 | 52 | 259 | 12 |
| 2003 | Hessels | Netherlands/Europe | Caucasian | NR | NR | PCR | Urine | cutoff 0.2 | 24 | 84 | 16 | 14 | 8 | 70 | 11 |
median;
95%CI;
IQR (interquartile range);
missing;
SEM;
area under curve;
PCA3/PSA
data are not available; mean median (ranges); cutoff values were not provided because these studies found serum PCA3 has no correlation with PCa.
Quantitative synthesis results
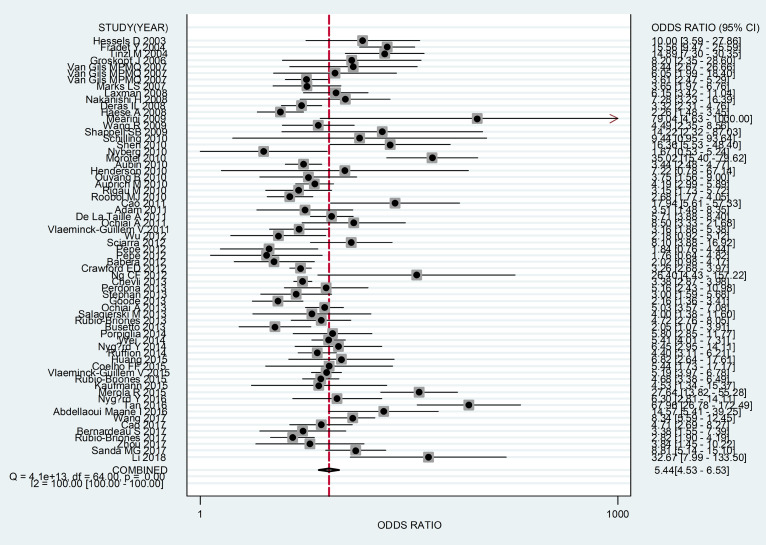
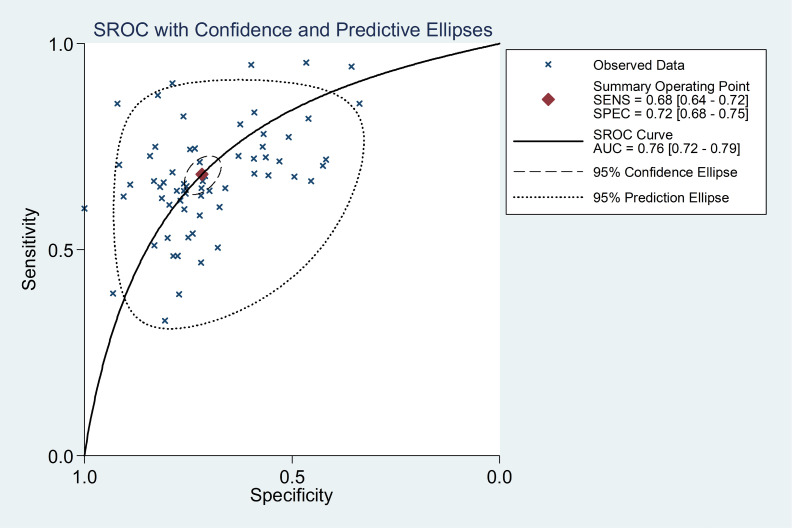
In this meta-analysis, the random-effects model was selected to calculate the sensitivity, specificity, LR+, and LR− with corresponding 95% CIs, because of the obvious between-study heterogeneity among those studies (P <0.05). The meta—analytic results showed that the pooled overall diagnostic sensitivity, specificity, LR+, LR− and 95% CIs about PCA3 for predicting significant PCa were 0.68 (0.64-0.72), 0.72 (0.68-0.75), 2.41 (2.16-2.69), 0.44 (0.40-0.49), respectively (Figure-2). Moreover, the summary diagnostic odds ratio (DOR) and 95% CIs for the diagnostic value of PCA3 in PCa patients was 5.44 (4.53-6.53) (Figure-3). In addition, AUC and 95% CI was 0.76 (0.72-0.79) (Figure-4).
Figure 2. Flowchart of literature search and selection process.
Figure 3. Forest plots of summary diagnostic odds ratio of by PCA3 as a diagnostic marker for PCa in this meta-analysis. Each solid circle represents an eligible study. The size of solid circle reflects the sample size of each eligible study. Error bars represent 95% CIs.
Figure 4. Summary receiver operating characteristic curves of PCA3 for the diagnosis of PCa. Each solid circle represents an eligible study. The size of solid circle represents the sample size of each eligible study. The overall diagnostic efficiency is summarized by the regression curve.
Test of heterogeneity
The I2-square of sensitivity, specificity, LR+, LR− and DOR in this meta-analysis were as follows: 88.86%, 92.08%, 82.17%, 81.70% and 100%, which proved that the heterogeneity between eligible studies was significant. As a result, the random effects model was chosen to synthesize the relevant data mentioned above.
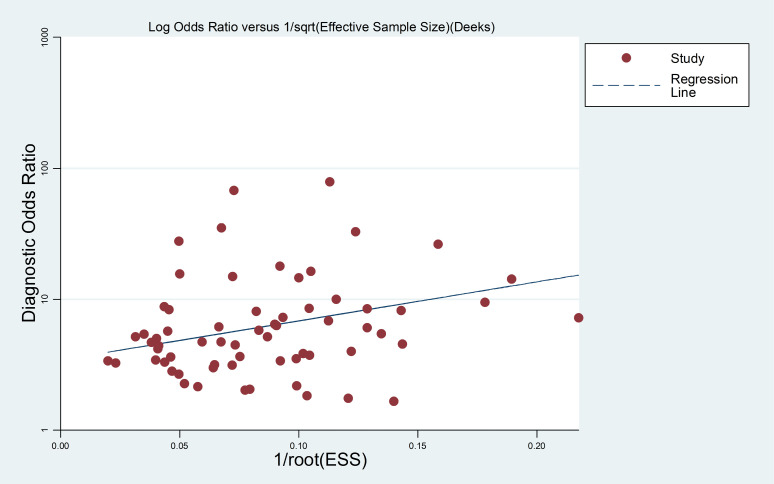
Publication bias
The potential publication bias of the included studies was evaluated through the Deek's funnel plot asymmetry test. The data of the slope coefficient of the regression line were symmetric, which suggested that the meta-analysis did not have a likelihood of publication bias (Figure-5).
Figure 5. Linear regression test of funnel plot asymmetry. The statistically non-significant P value of the slop coefficient indicates symmetry of the data and a low likelihood of publication bias.
DISCUSSION
Though PCa presents a slow progress, it has become a big threat to the health of men (4). Thus, the intervention at the early staging of PCa improves clinical prognosis. Serum PSA, DRE and transrectal ultrasound are still served as the screening of PCa in many countries and areas, which provides clinicians a low positive rate in the diagnosis of PCa (8). Among them, PSA is a serum marker widely used for screening of PCa in past years (4, 7). However, the proportion of positive biopsy is less than 50% in men with elevated serum PSA (10, 77). Therefore, the false-positive of PSA results may lead to unnecessary prostate biopsies and cause the complications of prostate biopsy (78). For these reasons, the searching for novel specific biomarkers of PCa has been attempted all the time.
In recent years, several serologic and pathologic biomarkers, with higher specificity than serum PSA, have been found to reduce unnecessary biopsy and inform the treatment (79, 80). Among them, PCA3 is one of the most valuable biomarkers in the detection of PCa (80). There are different expression of PCA3 gene in PCa tissue and other noncancerous tissue, which provides a great help for clinician to distinguish PCa from other prostatic diseases (80, 81). PCA3 gene is located on the long arm of chromosome 9 with 23kb long of nucleic acid and four exons and it cannot be translated into protein in normal cells (11, 82). In addition, it is a specific biomarker, over-expressed in more than 95% of PCa cells, so it can help to distinguish benign from cancerous prostate cells with an accuracy approaching 100% (83). Besides, PCA3 is also not affected by age, prostate volume or other prostatic diseases (81). In clinic, it is normally extracted in urine samples collected after DRE (11). And PROGENSA PCA3 assay has been already widely used to measure the level of urinary PCA3, and it can also been measured in serum and tissue samples (20, 23, 84).
Over the past years, many studies have increased to evaluate the value of PCA3 in the detection of PCa. In order to elucidate the expression differences of PCA3, meta-analysis has been updated to comprehensively and systematically investigate the diagnosis accuracy of PCA3 level in PCa patients. However, the outcomes of these studies remained inconsistent and controversial. There were several variables in these studies, such as the different ethnicities, the small sample size of individual study, the possible limited effect of individual patient data, among other factors, which could have caused the limited statistical power in the published studies. Compared with previous review and meta—analysis (85-87), this meta-analysis contains more studies for the sake of the sufficient evidence of our results. Furthermore, the publication of the previous meta-analysis might generate great influence on the results. All these factors made contributions to the development of the current meta-analysis.
Compared to a single study, meta-analysis would provide more sufficient results. Thus, we suggested that there existed stronger advantages to prove the relevance between the level of PCA3 and the diagnosis of PCa. Though it was deemed that PCA3 might be a valuable diagnostic biomarker of PCa in the previous studies, correlation between PCA3 level and the diagnosis of PCa remains unclear. Therefore, we need a better method for further analysis and elaboration about the diagnostic value of PCA3 for PCa. In the present meta-analysis, the summary DOR and 95% CIs for PCA3 was 5.44 (4.53-6.53), and AUC and 95% CIs was 0.76 (0.72-0.79). Thus, the above results revealed that PCA3 could be acceptable as a valuable biomarker to distinguish PCa patients from healthy individuals.
Overall, the sufficient statistical evidences including the large sample size were used to estimate the diagnostic value of PCA3 in the detection of PCa. However, several limitations were involved in this meta-analysis. First of all, the ethnicities involved in these studies were mainly Caucasians, However, Asian and African populations were included in relatively few studies. Thus, more attention should be paid to the influence of ethnicity. Secondly, there was a threshold effect and obvious heterogeneity in this meta-analysis, probably due to the large difference in reagent resource, patient characteristics, the assay type and the cut-off value. Moreover, the lack of sufficient data, the internal references and cut-off values were not considered in meta-regression analysis. Hence, it might reduce the reliability of our meta-analysis. In addition, more attention should be paid in further researches to the comparison of PCA3, PSA, and other biomarkers in the diagnosis of PCa. To improve reliability of the meta-analysis, well-designed studies with large sample size should be continued to evaluate the effectiveness of PCA3 in the detection of PCa in the subsequent years.
CONCLUSIONS
This meta-analysis suggested that PCA3 is acceptable as a valuable diagnostic biomarker in the management of PCa, which is a non-invasive method with the acceptable sensitivity and specificity in the diagnosis of PCa to distinguish patients from healthy individuals. To further evaluate the diagnostic value of PCA3 in patients with PCa, more well-designed studies with large sample sizes are needed to validate the effectiveness of PCA3 to differentially diagnose PCa.
Glossary
ABBREVIATIONS
- PCA3
prostate cancer antigen 3;
- PCa
prostate cancer;
- LR+
positive likelihood ratios;
- LR−
negative likelihood ratios;
- CIs
confidence intervals;
- sROCs
ummary receiver operating characteristic;
- AUC
area under sROC curves;
- BPH
benign prostatic hyperplasia;
- PSA
prostate-specific antigen;
- TP
true positive;
- TN
true negative;
- FP
false positive;
- FN
false negative;
- DOR
diagnostic odds ratio;
- ELISA
enzyme-linked immunosorbent assay;
- RT-PCR
reverse transcription-polymerase chain reaction.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]; 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [DOI] [PubMed]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]; 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [DOI] [PubMed]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]; 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed]
- 4.Daniyal M, Siddiqui ZA, Akram M, Asif HM, Sultana S, Khan A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev. 2014;15:9575–9578. doi: 10.7314/apjcp.2014.15.22.9575. [DOI] [PubMed] [Google Scholar]; 4. Daniyal M, Siddiqui ZA, Akram M, Asif HM, Sultana S, Khan A. Epidemiology, etiology, diagnosis and treatment of prostate cancer. Asian Pac J Cancer Prev. 2014;15:9575-8. [DOI] [PubMed]
- 5.Filella X, Foj L. Prostate Cancer Detection and Prognosis: From Prostate Specific Antigen (PSA) to Exosomal Biomarkers. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111784. [DOI] [PMC free article] [PubMed] [Google Scholar]; 5. Filella X, Foj L. Prostate Cancer Detection and Prognosis: From Prostate Specific Antigen (PSA) to Exosomal Biomarkers. Int J Mol Sci. 2016;17. [DOI] [PMC free article] [PubMed]
- 6.Evangelista L, Zattoni F, Rossi E, Karnes RJ, Lowe V. Early detection of prostate cancer relapse by biochemistry and diagnostic imaging. Q J Nucl Med Mol Imaging. 2015;59:359–373. [PubMed] [Google Scholar]; 6. Evangelista L, Zattoni F, Rossi E, Karnes RJ, Lowe V. Early detection of prostate cancer relapse by biochemistry and diagnostic imaging. Q J Nucl Med Mol Imaging. 2015;59:359-73. [PubMed]
- 7.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]; 7. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-89. [DOI] [PubMed]
- 8.Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317:2532–2542. doi: 10.1001/jama.2017.7248. [DOI] [PubMed] [Google Scholar]; 8. Litwin MS, Tan HJ. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA. 2017;317:2532-2542. [DOI] [PubMed]
- 9.Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]; 9. Center MM, Jemal A, Lortet-Tieulent J, Ward E, Ferlay J, Brawley O, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079-92. [DOI] [PubMed]
- 10.Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311:1143–1149. doi: 10.1001/jama.2014.2085. [DOI] [PubMed] [Google Scholar]; 10. Hayes JH, Barry MJ. Screening for prostate cancer with the prostate-specific antigen test: a review of current evidence. JAMA. 2014;311:1143-9. [DOI] [PubMed]
- 11.Goode RR, Marshall SJ, Duff M, Chevli E, Chevli KK. Use of PCA3 in detecting prostate cancer in initial and repeat prostate biopsy patients. Prostate. 2013;73:48–53. doi: 10.1002/pros.22538. [DOI] [PubMed] [Google Scholar]; 11. Goode RR, Marshall SJ, Duff M, Chevli E, Chevli KK. Use of PCA3 in detecting prostate cancer in initial and repeat prostate biopsy patients. Prostate. 2013;73:48-53. [DOI] [PubMed]
- 12.Cao L, Lee CH, Ning J, Handy BC, Wagar EA, Meng QH. Combination of Prostate Cancer Antigen 3 and Prostate-Specific Antigen Improves Diagnostic Accuracy in Men at Risk of Prostate Cancer. Arch Pathol Lab Med. 2018;142:1106–1112. doi: 10.5858/arpa.2017-0185-OA. [DOI] [PubMed] [Google Scholar]; 12. Cao L, Lee CH, Ning J, Handy BC, Wagar EA, Meng QH. Combination of Prostate Cancer Antigen 3 and Prostate-Specific Antigen Improves Diagnostic Accuracy in Men at Risk of Prostate Cancer. Arch Pathol Lab Med. 2018;142:1106-12. [DOI] [PubMed]
- 13.Li M, Zhou D, Zhang W, Gao S, Zhou X. Urine PCA3 mRNA level in diagnostic of prostate cancer. J Cancer Res Ther. 2018;14:864–866. doi: 10.4103/jcrt.JCRT_734_17. [DOI] [PubMed] [Google Scholar]; 13. Li M, Zhou D, Zhang W, Gao S, Zhou X. Urine PCA3 mRNA level in diagnostic of prostate cancer. J Cancer Res Ther. 2018;14:864-6. [DOI] [PubMed]
- 14.Zhou Y, Li Y, Li X, Jiang M. Urinary Biomarker Panel to Improve Accuracy in Predicting Prostate Biopsy Result in Chinese Men with PSA 4-10 ng/mL. Biomed Res Int. 2017;2017 doi: 10.1155/2017/2512536. 2512536. [DOI] [PMC free article] [PubMed] [Google Scholar]; 14. Zhou Y, Li Y, Li X, Jiang M. Urinary Biomarker Panel to Improve Accuracy in Predicting Prostate Biopsy Result in Chinese Men with PSA 4-10 ng/mL. Biomed Res Int. 2017;2017:2512536. [DOI] [PMC free article] [PubMed]
- 15.Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan DW, et al. the EDRN-PCA3 Study Group. Bidair M, Kibel A, Lin DW, Lotan Y, Partin A, Taneja S. Association Between Combined TMPRSS2:ERG and PCA3 RNA Urinary Testing and Detection of Aggressive Prostate Cancer. JAMA Oncol. 2017;3:1085–1093. doi: 10.1001/jamaoncol.2017.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]; 15. Sanda MG, Feng Z, Howard DH, Tomlins SA, Sokoll LJ, Chan DW, et al. and the EDRN-PCA3 Study Group, Bidair M, Kibel A, Lin DW, Lotan Y, Partin A, Taneja S. Association Between Combined TMPRSS2:ERG and PCA3 RNA Urinary Testing and Detection of Aggressive Prostate Cancer. JAMA Oncol. 2017;3:1085-93. [DOI] [PMC free article] [PubMed]
- 16.Rubio-Briones J, Casanova J, Martínez F, Domínguez-Escrig JL, Fernández-Serra A, Dumont R, et al. PCA3 as a second-line biomarker in a prospective controlled randomized opportunistic prostate cancer screening programme. Actas Urol Esp. 2017;41:300–308. doi: 10.1016/j.acuro.2016.10.008. [DOI] [PubMed] [Google Scholar]; 16. Rubio-Briones J, Casanova J, Martínez F, Domínguez-Escrig JL, Fernández-Serra A, Dumont R, et al. PCA3 as a second-line biomarker in a prospective controlled randomized opportunistic prostate cancer screening programme. Actas Urol Esp. 2017;41:300-8. [DOI] [PubMed]
- 17.Bernardeau S, Charles T, Fromont-Hankard G, Irani J. The role of a single PCA3 test before a first negative prostate biopsy: 5-year follow-up. Prog Urol. 2017;27:325–330. doi: 10.1016/j.purol.2017.02.006. [DOI] [PubMed] [Google Scholar]; 17. Bernardeau S, Charles T, Fromont-Hankard G, Irani J. The role of a single PCA3 test before a first negative prostate biopsy: 5-year follow-up. Prog Urol. 2017;27:325-30. [DOI] [PubMed]
- 18.Wang T, Qu X, Jiang J, Gao P, Zhao D, Lian X, et al. Diagnostic significance of urinary long non-coding PCA3 RNA in prostate cancer. Oncotarget. 2017;8:58577–58586. doi: 10.18632/oncotarget.17272. [DOI] [PMC free article] [PubMed] [Google Scholar]; 18. Wang T, Qu X, Jiang J, Gao P, Zhao D, Lian X, et al. Diagnostic significance of urinary long non-coding PCA3 RNA in prostate cancer. Oncotarget. 2017;8:58577-86. [DOI] [PMC free article] [PubMed]
- 19.Schueler S, Schuetz GM, Dewey M. The revised QUADAS-2 tool. Ann Intern Med. 2012;156:323–323. doi: 10.7326/0003-4819-156-4-201202210-00018. author reply 323-4. [DOI] [PubMed] [Google Scholar]; 19. Schueler S, Schuetz GM, Dewey M. The revised QUADAS-2 tool. Ann Intern Med. 2012;156:323; author reply 323-4. [DOI] [PubMed]
- 20.Tan SJ, Xu LW, Xu Z, Wu JP, Liang K, Jia RP. [The value of PHI/PCA3 in the early diagnosis of prostate cancer] Zhonghua Yi Xue Za Zhi. 2016;96:100–103. doi: 10.3760/cma.j.issn.0376-2491.2016.02.005. [DOI] [PubMed] [Google Scholar]; 20. Tan SJ, Xu LW, Xu Z, Wu JP, Liang K, Jia RP. [The value of PHI/PCA3 in the early diagnosis of prostate cancer]. Zhonghua Yi Xue Za Zhi. 2016;96:100-3. [DOI] [PubMed]
- 21.Nygård Y, Haukaas SA, Halvorsen OJ, Gravdal K, Frugård J, Akslen LA, et al. A positive Real-Time Elastography (RTE) combined with a Prostate Cancer Gene 3 (PCA3) score above 35 convey a high probability of intermediate- or high-risk prostate cancer in patient admitted for primary prostate biopsy. BMC Urol. 2016;16:39–39. doi: 10.1186/s12894-016-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; 21. Nygård Y, Haukaas SA, Halvorsen OJ, Gravdal K, Frugård J, Akslen LA, et al. A positive Real-Time Elastography (RTE) combined with a Prostate Cancer Gene 3 (PCA3) score above 35 convey a high probability of intermediate- or high-risk prostate cancer in patient admitted for primary prostate biopsy. BMC Urol. 2016;16:39. [DOI] [PMC free article] [PubMed]
- 22.Kaufmann S, Bedke J, Gatidis S, Hennenlotter J, Kramer U, Notohamiprodjo M, et al. Prostate cancer gene 3 (PCA3) is of additional predictive value in patients with PI-RADS grade III (intermediate) lesions in the MR-guided re-biopsy setting for prostate cancer. World J Urol. 2016;34:509–515. doi: 10.1007/s00345-015-1655-8. [DOI] [PubMed] [Google Scholar]; 22. Kaufmann S, Bedke J, Gatidis S, Hennenlotter J, Kramer U, Notohamiprodjo M, et al. Prostate cancer gene 3 (PCA3) is of additional predictive value in patients with PI-RADS grade III (intermediate) lesions in the MR-guided re-biopsy setting for prostate cancer. World J Urol. 2016;34:509-15. [DOI] [PubMed]
- 23.Abdellaoui Maane I, El Hadi H, Qmichou Z, Al Bouzidi A, Bakri Y, Sefrioui H, et al. Evaluation of Combined Quantification of PCA3 and AMACR Gene Expression for Molecular Diagnosis of Prostate Cancer in Moroccan Patients by RT-qPCR. Asian Pac J Cancer Prev. 2016;17:5229–5235. doi: 10.22034/APJCP.2016.17.12.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]; 23. Abdellaoui Maane I, El Hadi H, Qmichou Z, Al Bouzidi A, Bakri Y, Sefrioui H, et al. Evaluation of Combined Quantification of PCA3 and AMACR Gene Expression for Molecular Diagnosis of Prostate Cancer in Moroccan Patients by RT-qPCR. Asian Pac J Cancer Prev. 2016;17:5229-35. [DOI] [PMC free article] [PubMed]
- 24.Vlaeminck-Guillem V, Devonec M, Champetier D, Decaussi-Petrucci M, Paparel P, Perrin P, et al. Urinary PCA3 to predict prostate cancer in a cohort of 1015 patients. Prog Urol. 2015;25:1160–1168. doi: 10.1016/j.purol.2015.08.005. [DOI] [PubMed] [Google Scholar]; 24. Vlaeminck-Guillem V, Devonec M, Champetier D, Decaussi-Petrucci M, Paparel P, Perrin P, et al. Urinary PCA3 to predict prostate cancer in a cohort of 1015 patients. Prog Urol. 2015;25:1160-8. [DOI] [PubMed]
- 25.Rubio-Briones J, Borque A, Esteban LM, Casanova J, Fernandez-Serra A, Rubio L, et al. Optimizing the clinical utility of PCA3 to diagnose prostate cancer in initial prostate biopsy. BMC Cancer. 2015;15:633–633. doi: 10.1186/s12885-015-1623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; 25. Rubio-Briones J, Borque A, Esteban LM, Casanova J, Fernandez-Serra A, Rubio L, et al. Optimizing the clinical utility of PCA3 to diagnose prostate cancer in initial prostate biopsy. BMC Cancer. 2015;15:633. [DOI] [PMC free article] [PubMed]
- 26.Nygård Y, Haukaas SA, Eide GE, Halvorsen OJ, Gravdal K, Frugård J, et al. Prostate cancer antigen-3 (PCA3) and PCA3-based nomograms in the diagnosis of prostate cancer: an external validation of Hansen's nomogram on a Norwegian cohort. Scand J Urol. 2015;49:8–15. doi: 10.3109/21681805.2014.949841. [DOI] [PubMed] [Google Scholar]; 26. Nygård Y, Haukaas SA, Eide GE, Halvorsen OJ, Gravdal K, Frugård J, et al. Prostate cancer antigen-3 (PCA3) and PCA3-based nomograms in the diagnosis of prostate cancer: an external validation of Hansen's nomogram on a Norwegian cohort. Scand J Urol. 2015;49:8-15. [DOI] [PubMed]
- 27.Merola R, Tomao L, Antenucci A, Sperduti I, Sentinelli S, Masi S, et al. PCA3 in prostate cancer and tumor aggressiveness detection on 407 high-risk patients: a National Cancer Institute experience. J Exp Clin Cancer Res. 2015;34:15–15. doi: 10.1186/s13046-015-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; 27. Merola R, Tomao L, Antenucci A, Sperduti I, Sentinelli S, Masi S, et al. PCA3 in prostate cancer and tumor aggressiveness detection on 407 high-risk patients: a National Cancer Institute experience. J Exp Clin Cancer Res. 2015;34:15. [DOI] [PMC free article] [PubMed]
- 28.Huang J, Reilly KH, Zhang HZ, Wang HB. Clinical evaluation of prostate cancer gene 3 score in diagnosis among Chinese men with prostate cancer and benign prostatic hyperplasia. BMC Urol. 2015;15:118–118. doi: 10.1186/s12894-015-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; 28. Huang J, Reilly KH, Zhang HZ, Wang HB. Clinical evaluation of prostate cancer gene 3 score in diagnosis among Chinese men with prostate cancer and benign prostatic hyperplasia. BMC Urol. 2015;15:118. [DOI] [PMC free article] [PubMed]
- 29.Coelho FF, Guimarães FL, Cabral WL, Salles PG, Mateo EC, Nogueira e Nogueira LM, et al. Expression of PCA3 and PSA genes as a biomarker for differential diagnosis of nodular hyperplasia and prostate cancer. Genet Mol Res. 2015;14:13519–13531. doi: 10.4238/2015.October.28.13. [DOI] [PubMed] [Google Scholar]; 29. Coelho FF, Guimarães FL, Cabral WL, Salles PG, Mateo EC, Nogueira e Nogueira LM, et al. Expression of PCA3 and PSA genes as a biomarker for differential diagnosis of nodular hyperplasia and prostate cancer. Genet Mol Res. 2015;14:13519-31. [DOI] [PubMed]
- 30.Wei JT, Feng Z, Partin AW, Brown E, Thompson I, Sokoll L, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer. J Clin Oncol. 2014;32:4066–4072. doi: 10.1200/JCO.2013.52.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]; 30. Wei JT, Feng Z, Partin AW, Brown E, Thompson I, Sokoll L, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol. 2014;32:4066-72. [DOI] [PMC free article] [PubMed]
- 31.Ruffion A, Perrin P, Devonec M, Champetier D, Decaussin M, Paparel P, et al. Additional value of PCA3 density to predict initial prostate biopsy outcome. World J Urol. 2014;32:917–923. doi: 10.1007/s00345-014-1251-3. [DOI] [PubMed] [Google Scholar]; 31. Ruffion A, Perrin P, Devonec M, Champetier D, Decaussin M, Paparel P, et al. Additional value of PCA3 density to predict initial prostate biopsy outcome. World J Urol. 2014;32:917-23. [DOI] [PubMed]
- 32.Rubio-Briones J, Casanova J, Dumont R, Rubio L, Fernandez-Serra A, Casanova-Salas I, et al. Optimizing prostate cancer screening; prospective randomized controlled study of the role of PSA and PCA3 testing in a sequential manner in an opportunistic screening program. Actas Urol Esp. 2014;38:217–223. doi: 10.1016/j.acuro.2013.09.007. [DOI] [PubMed] [Google Scholar]; 32. Rubio-Briones J, Casanova J, Dumont R, Rubio L, Fernandez-Serra A, Casanova-Salas I, et al. Optimizing prostate cancer screening; prospective randomized controlled study of the role of PSA and PCA3 testing in a sequential manner in an opportunistic screening program. Actas Urol Esp. 2014;38:217-23. [DOI] [PubMed]
- 33.Porpiglia F, Russo F, Manfredi M, Mele F, Fiori C, Bollito E, et al. The roles of multiparametric magnetic resonance imaging, PCA3 and prostate health index-which is the best predictor of prostate cancer after a negative biopsy? J Urol. 2014;192:60–66. doi: 10.1016/j.juro.2014.01.030. [DOI] [PubMed] [Google Scholar]; 33. Porpiglia F, Russo F, Manfredi M, Mele F, Fiori C, Bollito E, et al. The roles of multiparametric magnetic resonance imaging, PCA3 and prostate health index-which is the best predictor of prostate cancer after a negative biopsy? J Urol. 2014;192:60-6. [DOI] [PubMed]
- 34.Chevli KK, Duff M, Walter P, Yu C, Capuder B, Elshafei A, et al. Urinary PCA3 as a predictor of prostate cancer in a cohort of 3,073 men undergoing initial prostate biopsy. J Urol. 2014;191:1743–1748. doi: 10.1016/j.juro.2013.12.005. [DOI] [PubMed] [Google Scholar]; 34. Chevli KK, Duff M, Walter P, Yu C, Capuder B, Elshafei A, et al. Urinary PCA3 as a predictor of prostate cancer in a cohort of 3,073 men undergoing initial prostate biopsy. J Urol. 2014;191:1743-8. [DOI] [PubMed]
- 35.Stephan C, Jung K, Semjonow A, Schulze-Forster K, Cammann H, Hu X, et al. Comparative assessment of urinary prostate cancer antigen 3 and TMPRSS2:ERG gene fusion with the serum [-2]proprostate-specific antigen-based prostate health index for detection of prostate cancer. Clin Chem. 2013;59:280–288. doi: 10.1373/clinchem.2012.195560. [DOI] [PubMed] [Google Scholar]; 35. Stephan C, Jung K, Semjonow A, Schulze-Forster K, Cammann H, Hu X, et al. Comparative assessment of urinary prostate cancer antigen 3 and TMPRSS2:ERG gene fusion with the serum [-2]proprostate-specific antigen-based prostate health index for detection of prostate cancer. Clin Chem. 2013;59:280-8. [DOI] [PubMed]
- 36.Salagierski M, Mulders P, Schalken JA. Predicting prostate biopsy outcome using a PCA3-based nomogram in a Polish cohort. Anticancer Res. 2013;33:553–557. [PubMed] [Google Scholar]; 36. Salagierski M, Mulders P, Schalken JA. Predicting prostate biopsy outcome using a PCA3-based nomogram in a Polish cohort. Anticancer Res. 2013;33:553-7. [PubMed]
- 37.Perdonà S, Bruzzese D, Ferro M, Autorino R, Marino A, Mazzarella C, et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve diagnostic accuracy in patients undergoing prostate biopsy. Prostate. 2013;73:227–235. doi: 10.1002/pros.22561. [DOI] [PubMed] [Google Scholar]; 37. Perdonà S, Bruzzese D, Ferro M, Autorino R, Marino A, Mazzarella C, et al. Prostate health index (phi) and prostate cancer antigen 3 (PCA3) significantly improve diagnostic accuracy in patients undergoing prostate biopsy. Prostate. 2013;73:227-35. [DOI] [PubMed]
- 38.Ochiai A, Okihara K, Kamoi K, Oikawa T, Shimazui T, Murayama S, et al. Clinical utility of the prostate cancer gene 3 (PCA3) urine assay in Japanese men undergoing prostate biopsy. BJU Int. 2013;111:928–933. doi: 10.1111/j.1464-410X.2012.11683.x. [DOI] [PubMed] [Google Scholar]; 38. Ochiai A, Okihara K, Kamoi K, Oikawa T, Shimazui T, Murayama S, et al. Clinical utility of the prostate cancer gene 3 (PCA3) urine assay in Japanese men undergoing prostate biopsy. BJU Int. 2013;111:928-33. [DOI] [PubMed]
- 39.Busetto GM, De Berardinis E, Sciarra A, Panebianco V, Giovannone R, Rosato S, et al. Prostate cancer gene 3 and multiparametric magnetic resonance can reduce unnecessary biopsies: decision curve analysis to evaluate predictive models. Urology. 2013;82:1355–1360. doi: 10.1016/j.urology.2013.06.078. [DOI] [PubMed] [Google Scholar]; 39. Busetto GM, De Berardinis E, Sciarra A, Panebianco V, Giovannone R, Rosato S, et al. Prostate cancer gene 3 and multiparametric magnetic resonance can reduce unnecessary biopsies: decision curve analysis to evaluate predictive models. Urology. 2013;82:1355-60. [DOI] [PubMed]
- 40.Wu AK, Reese AC, Cooperberg MR, Sadetsky N, Shinohara K. Utility of PCA3 in patients undergoing repeat biopsy for prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:100–105. doi: 10.1038/pcan.2011.52. [DOI] [PubMed] [Google Scholar]; 40. Wu AK, Reese AC, Cooperberg MR, Sadetsky N, Shinohara K. Utility of PCA3 in patients undergoing repeat biopsy for prostate cancer. Prostate Cancer Prostatic Dis. 2012;15:100-5. [DOI] [PubMed]
- 41.Sciarra A, Panebianco V, Cattarino S, Busetto GM, De Berardinis E, Ciccariello M, et al. Multiparametric magnetic resonance imaging of the rostate can improve the predictive value of the urinary prostate cancer antigen 3 test in patients with elevated prostate-specific antigen levels and a previous negative biopsy. BJU Int. 2012;110(11):1661–1665. doi: 10.1111/j.1464-410X.2012.11146.x. [DOI] [PubMed] [Google Scholar]; 41. Sciarra A, Panebianco V, Cattarino S, Busetto GM, De Berardinis E, Ciccariello M, et al. Multiparametric magnetic resonance imaging of the rostate can improve the predictive value of the urinary prostate cancer antigen 3 test in patients with elevated prostate-specific antigen levels and a previous negative biopsy. BJU Int. 2012;110(11):1661-5. [DOI] [PubMed]
- 42.Pepe P, Fraggetta F, Galia A, Skonieczny G, Aragona F. PCA3 score and prostate cancer diagnosis at repeated saturation biopsy. Which cut-off: 20 or 35? Int Braz J Urol. 2012;38:489–495. doi: 10.1590/s1677-55382012000400008. [DOI] [PubMed] [Google Scholar]; 42. Pepe P, Fraggetta F, Galia A, Skonieczny G, Aragona F. PCA3 score and prostate cancer diagnosis at repeated saturation biopsy. Which cut-off: 20 or 35? Int Braz J Urol. 2012;38:489-95. [DOI] [PubMed]
- 43.Ng CF, Yeung R, Chiu PK, Lam NY, Chow J, Chan B. The role of urine prostate cancer antigen 3 mRNA levels in the diagnosis of prostate cancer among Hong Kong Chinese patients. Hong Kong Med J. 2012;18:459–465. [PubMed] [Google Scholar]; 43. Ng CF, Yeung R, Chiu PK, Lam NY, Chow J, Chan B. The role of urine prostate cancer antigen 3 mRNA levels in the diagnosis of prostate cancer among Hong Kong Chinese patients. Hong Kong Med J. 2012;18:459-65. [PubMed]
- 44.Crawford ED, Rove KO, Trabulsi EJ, Qian J, Drewnowska K P, Kaminetsky JC, et al. Diagnostic performance of PCA3 to detect prostate cancer in men with increased prostate specific antigen: a prospective study of 1,962 cases. J Urol. 2012;188:1726–1731. doi: 10.1016/j.juro.2012.07.023. [DOI] [PubMed] [Google Scholar]; 44. Crawford ED, Rove KO, Trabulsi EJ, Qian J, Drewnowska K P, Kaminetsky JC, et al. Diagnostic performance of PCA3 to detect prostate cancer in men with increased prostate specific antigen: a prospective study of 1,962 cases. J Urol. 2012;188:1726-31. [DOI] [PubMed]
- 45.Barbera M, Pepe P, Paola Q, Aragona F. PCA3 score accuracy in diagnosing prostate cancer at repeat biopsy: our experience in 177 patients. Arch Ital Urol Androl. 2012;84:227–229. [PubMed] [Google Scholar]; 45. Barbera M, Pepe P, Paola Q, Aragona F. PCA3 score accuracy in diagnosing prostate cancer at repeat biopsy: our experience in 177 patients. Arch Ital Urol Androl. 2012;84:227-9. [PubMed]
- 46.Vlaeminck-Guillem V, Campos-Fernandes JL, Champetier D, Chikh K, Decaussin-Petrucci M, Devonec M, et al. Value of PCA3 urinary test for prostate biopsy decision: the Lyon-Sud University Hospital experience. Ann Biol Clin (Paris) 2011;69:31–39. doi: 10.1684/abc.2010.0513. [DOI] [PubMed] [Google Scholar]; 46. Vlaeminck-Guillem V, Campos-Fernandes JL, Champetier D, Chikh K, Decaussin-Petrucci M, Devonec M, et al. Value of PCA3 urinary test for prostate biopsy decision: the Lyon-Sud University Hospital experience. Ann Biol Clin (Paris). 2011;69:31-9. [DOI] [PubMed]
- 47.Shen M, Chen W, Yu K, Chen Z, Zhou W, Lin X, et al. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp Mol Pathol. 2011;90:97–100. doi: 10.1016/j.yexmp.2010.10.009. [DOI] [PubMed] [Google Scholar]; 47. Shen M, Chen W, Yu K, Chen Z, Zhou W, Lin X, et al. The diagnostic value of PCA3 gene-based analysis of urine sediments after digital rectal examination for prostate cancer in a Chinese population. Exp Mol Pathol. 2011;90:97-100. [DOI] [PubMed]
- 48.Pepe P, Aragona F. PCA3 score vs PSA free/total accuracy in prostate cancer diagnosis at repeat saturation biopsy. Anticancer Res. 2011;31:4445–4449. [PubMed] [Google Scholar]; 48. Pepe P, Aragona F. PCA3 score vs PSA free/total accuracy in prostate cancer diagnosis at repeat saturation biopsy. Anticancer Res. 2011;31:4445-9. [PubMed]
- 49.Ochiai A, Okihara K, Kamoi K, Iwata T, Kawauchi A, Miki T, et al. Prostate cancer gene 3 urine assay for prostate cancer in Japanese men undergoing prostate biopsy. Int J Urol. 2011;18:200–205. doi: 10.1111/j.1442-2042.2010.02711.x. [DOI] [PubMed] [Google Scholar]; 49. Ochiai A, Okihara K, Kamoi K, Iwata T, Kawauchi A, Miki T, et al. Prostate cancer gene 3 urine assay for prostate cancer in Japanese men undergoing prostate biopsy. Int J Urol. 2011;18:200-5. [DOI] [PubMed]
- 50.de la Taille A, Irani J, Graefen M, Chun F, de Reijke T, Kil P, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol. 2011;185:2119–2125. doi: 10.1016/j.juro.2011.01.075. [DOI] [PubMed] [Google Scholar]; 50. de la Taille A, Irani J, Graefen M, Chun F, de Reijke T, Kil P, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol. 2011;185:2119-25. [DOI] [PubMed]
- 51.Cao DL, Ye DW, Zhang HL, Zhu Y, Wang YX, Yao XD. A multiplex model of combining gene-based, protein-based, and metabolite-based with positive and negative markers in urine for the early diagnosis of prostate cancer. Prostate. 2011;71:700–710. doi: 10.1002/pros.21286. [DOI] [PubMed] [Google Scholar]; 51. Cao DL, Ye DW, Zhang HL, Zhu Y, Wang YX, Yao XD. A multiplex model of combining gene-based, protein-based, and metabolite-based with positive and negative markers in urine for the early diagnosis of prostate cancer. Prostate. 2011;71:700-10. [DOI] [PubMed]
- 52.Adam A, Engelbrecht MJ, Bornman MS, Manda SO, Moshokoa E, Feilat RA. The role of the PCA3 assay in predicting prostate biopsy outcome in a South African setting. BJU Int. 2011;108:1728–1733. doi: 10.1111/j.1464-410X.2011.10202.x. [DOI] [PubMed] [Google Scholar]; 52. Adam A, Engelbrecht MJ, Bornman MS, Manda SO, Moshokoa E, Feilat RA. The role of the PCA3 assay in predicting prostate biopsy outcome in a South African setting. BJU Int. 2011;108:1728-33. [DOI] [PubMed]
- 53.Schilling D, Hennenlotter J, Munz M, Bökeler U, Sievert KD, Stenzl A. Interpretation of the prostate cancer gene 3 in reference to the individual clinical background: implications for daily practice. Urol Int. 2010;85:159–165. doi: 10.1159/000314078. [DOI] [PubMed] [Google Scholar]; 53. Schilling D, Hennenlotter J, Munz M, Bökeler U, Sievert KD, Stenzl A. Interpretation of the prostate cancer gene 3 in reference to the individual clinical background: implications for daily practice. Urol Int. 2010;85:159-65. [DOI] [PubMed]
- 54.Roobol MJ, Schröder FH, van Leeuwen P, Wolters T, van den Bergh RC, van Leenders GJ, et al. Performance of the prostate cancer antigen 3 (PCA3) gene and prostate-specific antigen in prescreened men: exploring the value of PCA3 for a first-line diagnostic test. Eur Urol. 2010;58:475–481. doi: 10.1016/j.eururo.2010.06.039. [DOI] [PubMed] [Google Scholar]; 54. Roobol MJ, Schröder FH, van Leeuwen P, Wolters T, van den Bergh RC, van Leenders GJ, et al. Performance of the prostate cancer antigen 3 (PCA3) gene and prostate-specific antigen in prescreened men: exploring the value of PCA3 for a first-line diagnostic test. Eur Urol. 2010;58:475-81. [DOI] [PubMed]
- 55.Rigau M, Morote J, Mir MC, Ballesteros C, Ortega I, Sanchez A, et al. PSGR and PCA3 as biomarkers for the detection of prostate cancer in urine. Prostate. 2010;70:1760–1767. doi: 10.1002/pros.21211. [DOI] [PubMed] [Google Scholar]; 55. Rigau M, Morote J, Mir MC, Ballesteros C, Ortega I, Sanchez A, et al. PSGR and PCA3 as biomarkers for the detection of prostate cancer in urine. Prostate. 2010;70:1760-7. [DOI] [PubMed]
- 56.Nyberg M, Ulmert D, Lindgren A, Lindström U, Abrahamsson PA, Bjartell A. PCA3 as a diagnostic marker for prostate cancer: a validation study on a Swedish patient population. Scand J Urol Nephrol. 2010;44:378–383. doi: 10.3109/00365599.2010.521187. [DOI] [PubMed] [Google Scholar]; 56. Nyberg M, Ulmert D, Lindgren A, Lindström U, Abrahamsson PA, Bjartell A. PCA3 as a diagnostic marker for prostate cancer: a validation study on a Swedish patient population. Scand J Urol Nephrol. 2010;44:378-83. [DOI] [PubMed]
- 57.Morote J, Rigau M, Garcia M, Mir C, Ballesteros C, Planas J, et al. Behavior of the PCA3 gene in the urine of men with high grade prostatic intraepithelial neoplasia. World J Urol. 2010;28:677–680. doi: 10.1007/s00345-010-0580-0. [DOI] [PubMed] [Google Scholar]; 57. Morote J, Rigau M, Garcia M, Mir C, Ballesteros C, Planas J, et al. Behavior of the PCA3 gene in the urine of men with high grade prostatic intraepithelial neoplasia. World J Urol. 2010;28:677-80. [DOI] [PubMed]
- 58.Henderson J, Ghani KR, Cook J, Fahey M, Schalken J, Thilagarajah R. The role of PCA3 testing in patients with a raised prostate-specific antigen level after Greenlight photoselective vaporization of the prostate. J Endourol. 2010;24:1821–1824. doi: 10.1089/end.2010.0196. [DOI] [PubMed] [Google Scholar]; 58. Henderson J, Ghani KR, Cook J, Fahey M, Schalken J, Thilagarajah R. The role of PCA3 testing in patients with a raised prostate-specific antigen level after Greenlight photoselective vaporization of the prostate. J Endourol. 2010;24:1821-4. [DOI] [PubMed]
- 59.Auprich M, Haese A, Walz J, Pummer K, de la Taille A, Graefen M, et al. External validation of urinary PCA3-based nomograms to individually predict prostate biopsy outcome. Eur Urol. 2010;58:727–732. doi: 10.1016/j.eururo.2010.06.038. [DOI] [PubMed] [Google Scholar]; 59. Auprich M, Haese A, Walz J, Pummer K, de la Taille A, Graefen M, et al. External validation of urinary PCA3-based nomograms to individually predict prostate biopsy outcome. Eur Urol. 2010;58:727-32. [DOI] [PubMed]
- 60.Aubin SM, Reid J, Sarno MJ, Blase A, Aussie J, Rittenhouse H, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol. 2010;184:1947–1952. doi: 10.1016/j.juro.2010.06.098. [DOI] [PubMed] [Google Scholar]; 60. Aubin SM, Reid J, Sarno MJ, Blase A, Aussie J, Rittenhouse H, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol. 2010;184:1947-52. [DOI] [PubMed]
- 61.Wang R, Chinnaiyan AM, Dunn RL, Wojno KJ, Wei JT. Rational approach to implementation of prostate cancer antigen 3 into clinical care. Cancer. 2009;115:3879–3886. doi: 10.1002/cncr.24447. [DOI] [PMC free article] [PubMed] [Google Scholar]; 61. Wang R, Chinnaiyan AM, Dunn RL, Wojno KJ, Wei JT. Rational approach to implementation of prostate cancer antigen 3 into clinical care. Cancer. 2009;115:3879-86. [DOI] [PMC free article] [PubMed]
- 62.Shappell SB, Fulmer J, Arguello D, Wright BS, Oppenheimer JR, Putzi MJ. PCA3 urine mRNA testing for prostate carcinoma: patterns of use by community urologists and assay performance in reference laboratory setting. Urology. 2009;73:363–368. doi: 10.1016/j.urology.2008.08.459. [DOI] [PubMed] [Google Scholar]; 62. Shappell SB, Fulmer J, Arguello D, Wright BS, Oppenheimer JR, Putzi MJ. PCA3 urine mRNA testing for prostate carcinoma: patterns of use by community urologists and assay performance in reference laboratory setting. Urology. 2009;73:363-8. [DOI] [PubMed]
- 63.Ouyang B, Bracken B, Burke B, Chung E, Liang J, Ho SM. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol. 2009;181:2508–2513. doi: 10.1016/j.juro.2009.01.110. discussion 2513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; 63. Ouyang B, Bracken B, Burke B, Chung E, Liang J, Ho SM. A duplex quantitative polymerase chain reaction assay based on quantification of alpha-methylacyl-CoA racemase transcripts and prostate cancer antigen 3 in urine sediments improved diagnostic accuracy for prostate cancer. J Urol. 2009;181:2508-13; discussion 2513-4. [DOI] [PMC free article] [PubMed]
- 64.Mearini E, Antognelli C, Del Buono C, Cochetti G, Giannantoni A, Nardelli E, et al. The combination of urine DD3(PCA3) mRNA and PSA mRNA as molecular markers of prostate cancer. Biomarkers. 2009;14:235–243. doi: 10.1080/13547500902807306. [DOI] [PubMed] [Google Scholar]; 64. Mearini E, Antognelli C, Del Buono C, Cochetti G, Giannantoni A, Nardelli E, et al. The combination of urine DD3(PCA3) mRNA and PSA mRNA as molecular markers of prostate cancer. Biomarkers. 2009;14:235-43. [DOI] [PubMed]
- 65.van Gils MP, Hessels D, Hulsbergen-van de Kaa CA, Witjes JA, Jansen CF, Mulders PF, et al. Detailed analysis of histopathological parameters in radical prostatectomy specimens and PCA3 urine test results. Prostate. 2008;68:1215–1222. doi: 10.1002/pros.20781. [DOI] [PubMed] [Google Scholar]; 65. van Gils MP, Hessels D, Hulsbergen-van de Kaa CA, Witjes JA, Jansen CF, Mulders PF, et al. Detailed analysis of histopathological parameters in radical prostatectomy specimens and PCA3 urine test results. Prostate. 2008;68:1215-22. [DOI] [PubMed]
- 66.Nakanishi H, Groskopf J, Fritsche HA, Bhadkamkar V, Blase A, Kumar SV, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804–1809. doi: 10.1016/j.juro.2008.01.013. [DOI] [PubMed] [Google Scholar]; 66. Nakanishi H, Groskopf J, Fritsche HA, Bhadkamkar V, Blase A, Kumar SV, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol. 2008;179:1804-9. [DOI] [PubMed]
- 67.Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645–649. doi: 10.1158/0008-5472.CAN-07-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]; 67. Laxman B, Morris DS, Yu J, Siddiqui J, Cao J, Mehra R, et al. A first-generation multiplex biomarker analysis of urine for the early detection of prostate cancer. Cancer Res. 2008;68:645-9. [DOI] [PMC free article] [PubMed]
- 68.Haese A, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol. 2008;54:1081–1088. doi: 10.1016/j.eururo.2008.06.071. [DOI] [PubMed] [Google Scholar]; 68. Haese A, de la Taille A, van Poppel H, Marberger M, Stenzl A, Mulders PF, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol. 2008;54:1081-8. [DOI] [PubMed]
- 69.Deras IL, Aubin SM, Blase A, Day JR, Koo S, Partin AW, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179:1587–1592. doi: 10.1016/j.juro.2007.11.038. [DOI] [PubMed] [Google Scholar]; 69. Deras IL, Aubin SM, Blase A, Day JR, Koo S, Partin AW, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol. 2008;179:1587-92. [DOI] [PubMed]
- 70.van Gils M P, Hessels D, van Hooij O, Jannink SA, Peelen WP, Hanssen SL, et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin Cancer Res. 2007;13:939–943. doi: 10.1158/1078-0432.CCR-06-2679. [DOI] [PubMed] [Google Scholar]; 70. van Gils M P, Hessels D, van Hooij O, Jannink SA, Peelen WP, Hanssen SL, et al. The time-resolved fluorescence-based PCA3 test on urinary sediments after digital rectal examination; a Dutch multicenter validation of the diagnostic performance. Clin Cancer Res. 2007;13:939-43. [DOI] [PubMed]
- 71.van Gils M P, Cornel EB, Hessels D, Peelen W P, Witjes JA, Mulders PF, et al. Molecular PCA3 diagnostics on prostatic fluid. Prostate. 2007;67:881–887. doi: 10.1002/pros.20564. [DOI] [PubMed] [Google Scholar]; 71. van Gils M P, Cornel EB, Hessels D, Peelen W P, Witjes JA, Mulders PF, et al. Molecular PCA3 diagnostics on prostatic fluid. Prostate. 2007;67:881-7. [DOI] [PubMed]
- 72.Marks LS, Fradet Y, Deras IL, Blase A, Mathis J, Aubin SM, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532–535. doi: 10.1016/j.urology.2006.12.014. [DOI] [PubMed] [Google Scholar]; 72. Marks LS, Fradet Y, Deras IL, Blase A, Mathis J, Aubin SM, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology. 2007;69:532-5. [DOI] [PubMed]
- 73.Groskopf J, Aubin SM, Deras IL, Blase A, Bodrug S, Clark C, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089–1095. doi: 10.1373/clinchem.2005.063289. [DOI] [PubMed] [Google Scholar]; 73. Groskopf J, Aubin SM, Deras IL, Blase A, Bodrug S, Clark C, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem. 2006;52:1089-95. [DOI] [PubMed]
- 74.Tinzl M, Marberger M, Horvath S, Chypre C. DD3PCA3 RNA analysis in urine--a new perspective for detecting prostate cancer. Eur Urol. 2004;46:182–186. doi: 10.1016/j.eururo.2004.06.004. discussion 187. [DOI] [PubMed] [Google Scholar]; 74. Tinzl M, Marberger M, Horvath S, Chypre C. DD3PCA3 RNA analysis in urine--a new perspective for detecting prostate cancer. Eur Urol. 2004;46:182-6; discussion 187. [DOI] [PubMed]
- 75.Fradet Y, Saad F, Aprikian A, Dessureault J, Elhilali M, Trudel C, et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology. 2004;64:311–315. doi: 10.1016/j.urology.2004.03.052. [DOI] [PubMed] [Google Scholar]; 75. Fradet Y, Saad F, Aprikian A, Dessureault J, Elhilali M, Trudel C, et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology. 2004;64:311-5. [DOI] [PubMed]
- 76.Hessels D, Klein Gunnewiek JM, van Oort I, Karthaus HF, van Leenders GJ, van Balken B, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol. 2003;44:8–15. doi: 10.1016/s0302-2838(03)00201-x. discussion 15-6. [DOI] [PubMed] [Google Scholar]; 76. Hessels D, Klein Gunnewiek JM, van Oort I, Karthaus HF, van Leenders GJ, van Balken B, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol. 2003;44:8-15; discussion 15-6. [DOI] [PubMed]
- 77.Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]; 77. Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70-98. [DOI] [PubMed]
- 78.Pinkhasov GI, Lin YK, Palmerola R, Smith P, Mahon F, Kaag MG, et al. Complications following prostate needle biopsy requiring hospital admission or emergency department visits - experience from 1000 consecutive cases. BJU Int. 2012;110:369–374. doi: 10.1111/j.1464-410X.2011.10926.x. [DOI] [PubMed] [Google Scholar]; 78. Pinkhasov GI, Lin YK, Palmerola R, Smith P, Mahon F, Kaag MG, et al. Complications following prostate needle biopsy requiring hospital admission or emergency department visits - experience from 1000 consecutive cases. BJU Int. 2012;110:369-74. [DOI] [PubMed]
- 79.Rodriguez J F, Eggener SE. Prostate Cancer and the Evolving Role of Biomarkers in Screening and Diagnosis. Radiol Clin North Am. 2018;56:187–196. doi: 10.1016/j.rcl.2017.10.002. [DOI] [PubMed] [Google Scholar]; 79. Rodriguez J F, Eggener SE. Prostate Cancer and the Evolving Role of Biomarkers in Screening and Diagnosis. Radiol Clin North Am. 2018;56:187-96. [DOI] [PubMed]
- 80.Smits M, Mehra N, Sedelaar M, Gerritsen W, Schalken JA. Molecular biomarkers to guide precision medicine in localized prostate cancer. Expert Rev Mol Diagn. 2017;17:791–804. doi: 10.1080/14737159.2017.1345627. [DOI] [PubMed] [Google Scholar]; 80. Smits M, Mehra N, Sedelaar M, Gerritsen W, Schalken JA. Molecular biomarkers to guide precision medicine in localized prostate cancer. Expert Rev Mol Diagn. 2017;17:791-804. [DOI] [PubMed]
- 81.Day JR, Jost M, Reynolds MA, Groskopf J, Rittenhouse H. PCA3: from basic molecular science to the clinical lab. Cancer Lett. 2011;301:1–6. doi: 10.1016/j.canlet.2010.10.019. [DOI] [PubMed] [Google Scholar]; 81. Day JR, Jost M, Reynolds MA, Groskopf J, Rittenhouse H. PCA3: from basic molecular science to the clinical lab. Cancer Lett. 2011;301:1-6. [DOI] [PubMed]
- 82.Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009;6:255–261. doi: 10.1038/nrurol.2009.40. [DOI] [PubMed] [Google Scholar]; 82. Hessels D, Schalken JA. The use of PCA3 in the diagnosis of prostate cancer. Nat Rev Urol. 2009;6:255-61. [DOI] [PubMed]
- 83.Marks LS, Bostwick DG. Prostate Cancer Specificity of PCA3 Gene Testing: Examples from Clinical Practice. Rev Urol. 2008;10:175–181. [PMC free article] [PubMed] [Google Scholar]; 83. Marks LS, Bostwick DG. Prostate Cancer Specificity of PCA3 Gene Testing: Examples from Clinical Practice. Rev Urol. 2008;10:175-81. [PMC free article] [PubMed]
- 84.Durand X, Moutereau S, Xylinas E, de la Taille A. Progensa™ PCA3 test for prostate cancer. Expert Rev Mol Diagn. 2011;11:137–144. doi: 10.1586/erm.10.122. [DOI] [PubMed] [Google Scholar]; 84. Durand X, Moutereau S, Xylinas E, de la Taille A. Progensa™ PCA3 test for prostate cancer. Expert Rev Mol Diagn. 2011;11:137-44. [DOI] [PubMed]
- 85.Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:25776–25776. doi: 10.1038/srep25776. [DOI] [PMC free article] [PubMed] [Google Scholar]; 85. Cui Y, Cao W, Li Q, Shen H, Liu C, Deng J, et al. Evaluation of prostate cancer antigen 3 for detecting prostate cancer: a systematic review and meta-analysis. Sci Rep. 2016;6:25776. [DOI] [PMC free article] [PubMed]
- 86.Luo Y, Gou X, Huang P, Mou C. Prostate cancer antigen 3 test for prostate biopsy decision: a systematic review and meta analysis. Chin Med J (Engl) 2014;127:1768–1774. [PubMed] [Google Scholar]; 86. Luo Y, Gou X, Huang P, Mou C. Prostate cancer antigen 3 test for prostate biopsy decision: a systematic review and meta analysis. Chin Med J (Engl). 2014;127:1768-74. [PubMed]
- 87.Xue WJ, Ying XL, Jiang JH, Xu YH. Prostate cancer antigen 3 as a biomarker in the urine for prostate cancer diagnosis: a meta-analysis. J Cancer Res Ther. 2014;10(Suppl):C218–C221. doi: 10.4103/0973-1482.145881. [DOI] [PubMed] [Google Scholar]; 87. Xue WJ, Ying XL, Jiang JH, Xu YH. Prostate cancer antigen 3 as a biomarker in the urine for prostate cancer diagnosis: a meta-analysis. J Cancer Res Ther. 2014;10(Suppl):C218-21. [DOI] [PubMed]