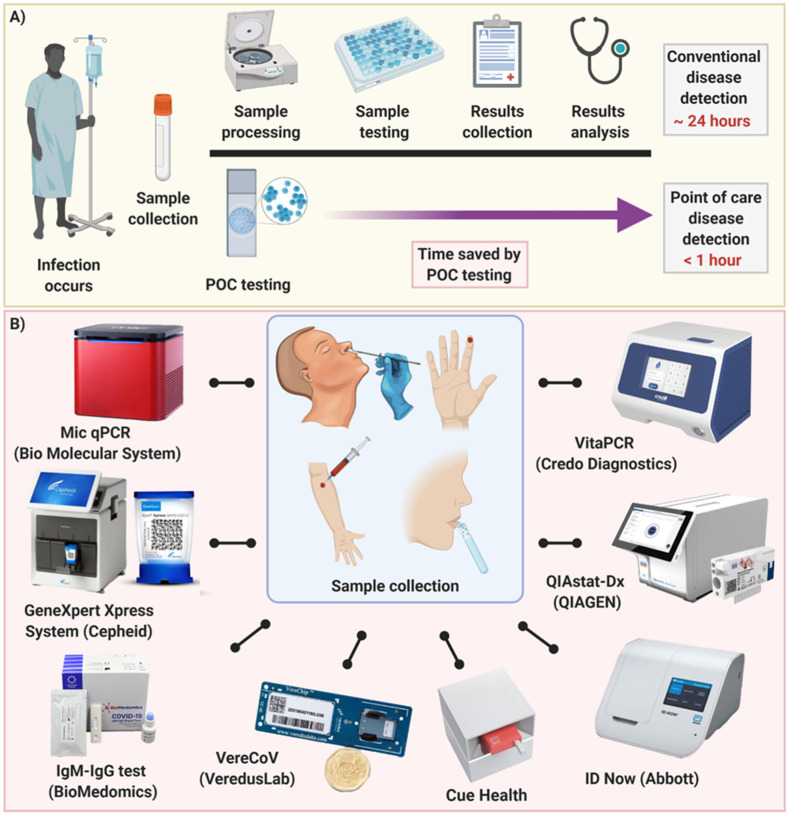
Figure 3.
(A) Schematic illustration of disease detection using conventional methods relied on centralized laboratories and POC testing approaches. POC devices can drastically reduce the amount of time needed to detect disease. (B) Current rapid commercially available POC devices that possess FDA approval for COVID-19. After sample collection and processing, these devices are capable of testing the sample in a time frame of mostly less than 30 min.