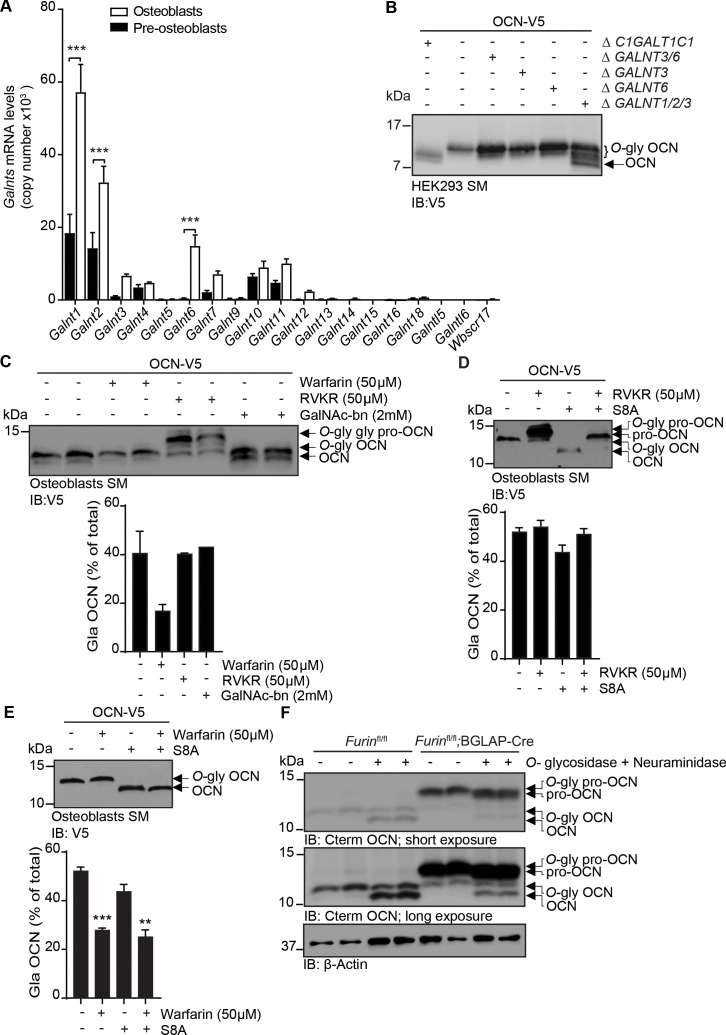
Figure 2. OCN O-glycosylation by N-acetylgalactosaminyltransferase (GalNAc-Ts) is independent of its processing and γ-carboxylation.
(A) Galnts expression in pre-osteoblasts (undifferentiated) and osteoblasts (differentiated) by quantitative PCR (n = 3 per condition). Results are represented as copy number of Galnts normalized to Actb. (B) Western blot analysis of OCN in the secretion media (SM) of HEK293 cells deficient for specific GalNAc-Ts. OCN-V5 was transfected in parental, C1GALT1C1-/- (Δ C1GALT1C1), or GALNTs deficient (Δ) HEK293 cells and analysed by western blot using anti-V5 antibody. (C) Western blot analysis on the SM of osteoblasts transfected with mouse OCN-V5 and treated or not with 2 mM of GalNAc-bn, 50 μM warfarin or 50 μM Dec-RVKR-CMK (RVKR) (upper panel), and percentage of carboxylated OCN (Gla-OCN) over total OCN measured by ELISA (lower panel; n = 2 per condition). (D) Western blot analysis on the SM of osteoblasts transfected with mouse OCN-V5 containing or not the S8A mutation and treated with 50 μM Dec-RVKR-CMK (RVKR) (upper panel), and percentage of carboxylated OCN (Gla-OCN) over total OCN measured by ELISA (lower panel; n = 3 per condition). (E) Western blot analysis on the SM of osteoblasts transfected with mouse OCN-V5 containing or not the S8A mutation and treated with 50 μM warfarin (upper panel), and percentage of carboxylated OCN over total OCN measured by ELISA (lower panel; n = 3 per condition). (F) Western blot analysis of OCN deglycosylation assay on bone extracts from Furinfl/fl and Furinfl/fl;BGLAP-Cre mice (n = 2 independent mice per genotype). Bone extracts were treated or not with O-glycosidase and neuraminidase for 4 hr at 37°C and analyzed by western blot using anti-C-termimal OCN antibody (Cterm OCN). **p<0.01; ***p<0.001 using one-way ANOVA with Bonferroni multiple comparisons test.
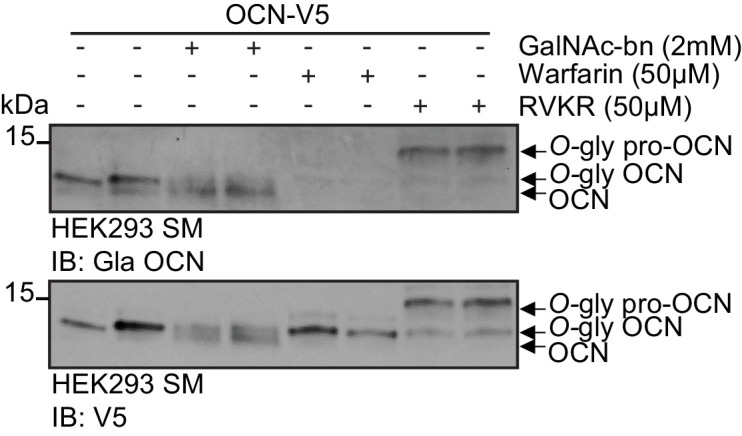
Figure 2—figure supplement 1. Mouse OCN O-glycosylation occurs independently of its carboxylation and processing in HEK293 cells.