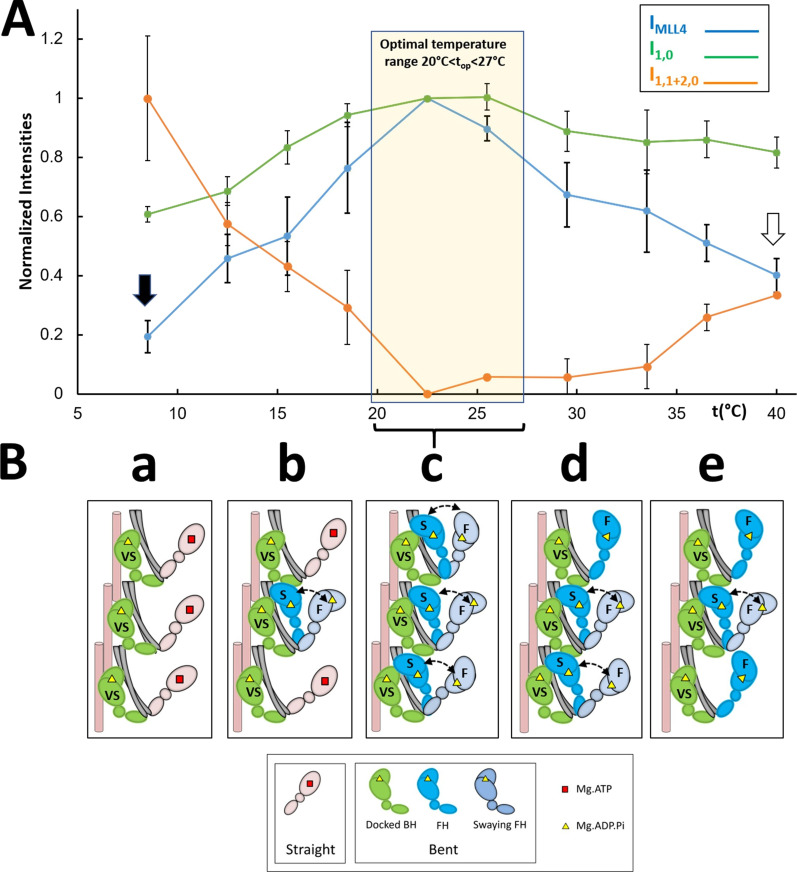
Figure 6.
Proposed thermosensing mechanism for thick filaments of relaxed tarantula skeletal muscle. (A) Normalized IMLL4, I1,0, and I1,1+2,0 (from Fig. 3, A–C). The residual values of IMLL4 are shown with black (20%) and white (40%) arrows. (B) Proposed thermosensing mechanism. (c) At the top range 20–27.5°C, the BHs (green) and FHs (blue) exhibit the bent structure required to form IHMs and helices. The FHs can sway away (light gray head, double arrow) and back, docking on their partner BH IHM positions. Lowering the temperature from this top range (b to a) progressively changes the heads to the straight structure (pink heads), incapable of making IHMs, and thus becoming disordered, which occurs in the FHs with slow (S) turnover rates, while the BHs with a very slow (VS) turnover rate remain ordered (c to b). We propose that progressively increasing temperature from the top range affects only a fraction of the FHs, which become semipermanently disordered midway to the thin filaments, while the BHs remain ordered (c to e; see text). The very slow (>1,800 s), slow (250–300 s), and fast (<30 s) ATP turnover rates measured in relaxed tarantula skeletal muscle thick filaments (Naber et al., 2011), associated with the docked BHs, docked FHs onto BHs, and released FHs, are marked in the heads as VS, S, and F, respectively (Alamo et al., 2016; see text). The results are given as mean ± SEM.