Figure 5.
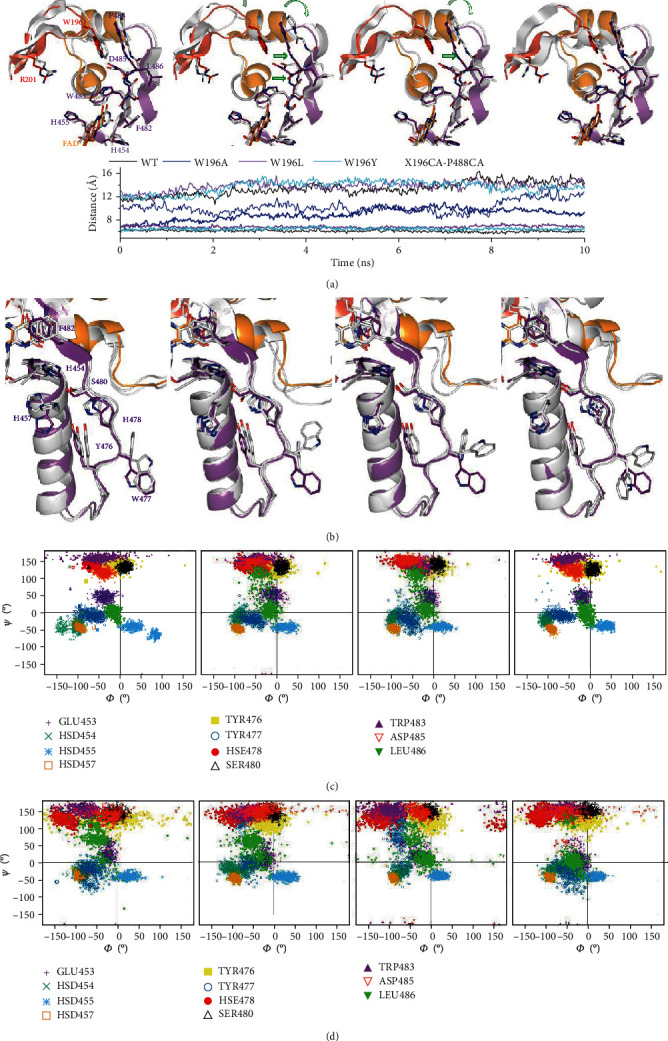
Impact of W196 hAIFΔ1-101 mutations on the dynamics of the β-hairpin and its environment. Representative conformations of the (a) β-hairpin and the central β-strand and the (b) His-rich helix and the loop connecting it to the central β-strand in final MD structures of selected replicates for each W196ox variant. MD final replicates are shown in grey in each panel. All panels compare to the crystallographic WTox structure (4BV6) that shows the β-hairpin in red, the C-loop in orange, and the rest of the protein in purple. Open green arrows indicate relevant displacements of structural elements relative to the crystallographic WTox structure. The lower panel in (a) shows the time evolution of the distances between Cαs of the residue at position 196 in the β-hairpin and P488 in the β-sheet. For each variant, data show averaged values for the 5 MD replicates run for each model in the hAIF∆1-101ox (bold lines) and CTC (line) states. Ramachandran plots of the distribution of key main chain Φ/ψ conformational along the MD of selected replicates for each (c) oxidized and (d) reduced W196 variant.
