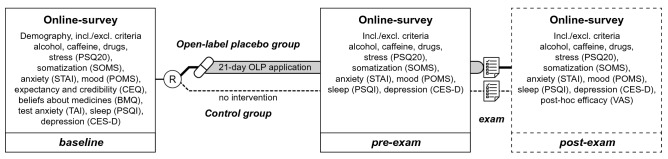
Figure 2.
Study design and assessments. The design comprised three online survey assessments: First, prior to randomization [R] (baseline), second, 3 days prior to the exam (pre-exam) and third, 60 days after the exam (post-exam). The latter additionally included the exam score, which was measured as percentage of correct answers in a standardized written midterm medical exam and served as primary outcome. Secondary, i.e. psychometric, outcomes were assessed by standardized questionnaires (PSQ20, Perceived Stress Questionnaire; SOMS, Screening for Somatoform Disorders; STAI, State-Trait-Anxiety Inventory; POMS, Profile of Mood States; CEQ, Credibility and Expectancy Questionnaire; BMQ, Beliefs about Medicines Questionnaire; TAI, Test-Anxiety Inventory; PSQI, Pittsburgh Sleep Quality Index; CES-D, Center for Epidemiologic Studies-Depression Scale; VAS, Visual Analogue Scale) via an online survey. [OLP, Open-label placebos].