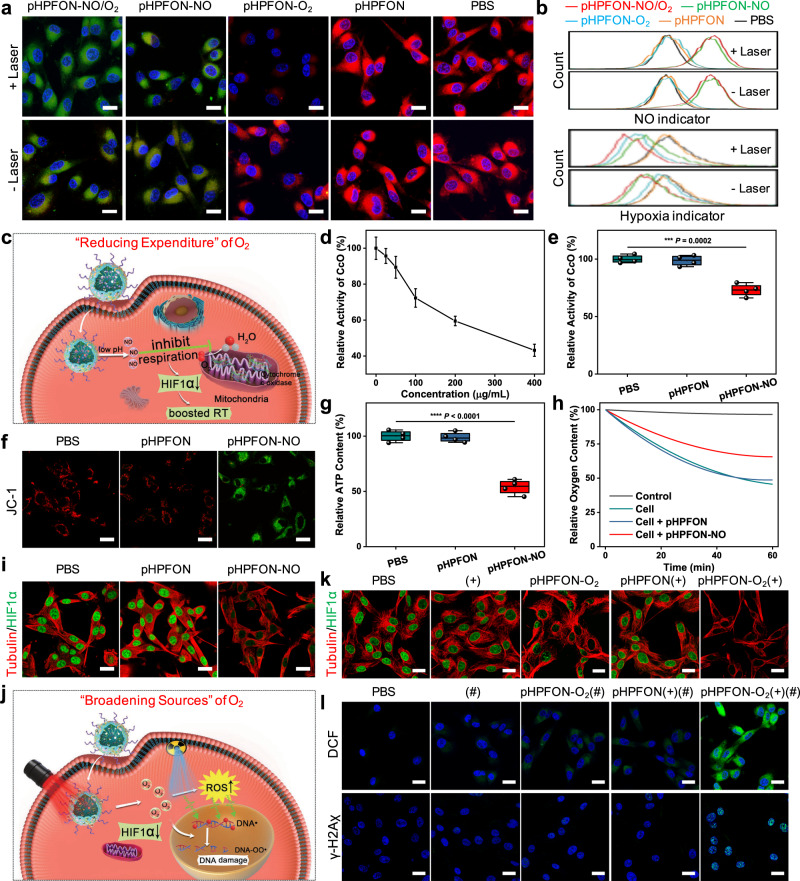
Fig. 4. In vitro programmable release and radiosensitizing effect of NO and O2.
a Confocal images of hypoxic U87MG cells treated with different pHPFON formulations for 24 h, with or without subsequent 808-nm laser irradiation (1 W/cm2, 3 min). Green, DAF-FM (4-amino-5-methylamino-2′,7′-difluorofluorescein, NO indicator). Red, [Ru(dpp)3]Cl2 (hypoxia indicator). Blue, DAPI. Scale bar, 20 µm. Experiments were performed three times with similar results. b Flow cytometry analysis of hypoxic U87MG cells receiving the same treatments in a. Experiments were performed twice with similar results. c Schematic illustration of the NO delivery-based “reducing expenditure” oxygenation strategy for boosted RT. The low-pH-induced NO release would inhibit mitochondrial respiration, downregulate HIF-1α expression, and boost RT efficacy. d Relative activity of cytochrome c oxidase (CcO) after incubating hypoxic U87MG cells with pHPFON-NO at different concentrations for 24 h. e–i Effect of cell respiration inhibition by the pHPFON-NO. e Relative CcO activity, f JC-1 assay (green, JC-1 monomer. Red, J-aggregates. Scale bar, 20 µm), g relative ATP contents, h oxygen consumption capacity, and i HIF-1α expression (green, HIF-1α; red, tubulin. Scale bar, 20 µm) after co-incubation of hypoxic U87MG cells with pHPFON-NO, pHPFON, or PBS overnight. j Schematic illustration of the O2 delivery-based “broadening sources” oxygenation strategy for advanced RT. The laser-activatable O2 release would downregulate HIF-1α expression and augment X-ray-induced oxidative DNA damage. k Anti-HIF-1α staining in hypoxic U87MG cells after different treatments. Green, HIF-1α; red, tubulin. Scale bar, 20 µm. l Evaluation of intracellular ROS generation and DNA damage with H2DCFDA assay and anti-γ-H2Aχ staining after different treatments. Green, 2′,7′-dichlorofluorescein (DCF) or γ-H2Aχ; blue, DAPI. Scale bar, 20 μm. For k, l, (+) stands for 808-nm laser irradiation at 1 W/cm2 for 3 min applied after 24 h of incubation with nanoparticles; (#) stands for 4-Gy X-ray irradiation following the laser irradiation, if applicable. n = 4 biologically independent samples per group (d, e, g). Experiments were performed three times with similar results (f, h, i, k, l). Data are presented as mean ± s.d. in d. For the boxplots, the middle line is the median, the lower and upper hinges correspond to the first and third quartiles, and whiskers represent ±1.5 interquartile range (e, g). Two-tailed Student’s t-test. ***P < 0.001. ****P < 0.0001.