Abstract
Purpose
To evaluate whether adjusting timing of modified natural cycle frozen embryo transfer (mNC-FET) 1 day earlier in the setting of a spontaneous LH surge has an impact on pregnancy outcomes.
Methods
This retrospective cohort study evaluated all mNC-FET with euploid blastocysts from May 1, 2016 to March 30, 2019, at a single academic institution. Standard protocol for mNC-FET included ultrasound monitoring and hCG trigger when the dominant follicle and endometrial lining were appropriately developed. Patients had serum LH, estradiol, and progesterone checked on day of trigger. If LH was ≥ 20 mIU/mL, trigger was given that day and FET was performed 6 days after surge (LH/HCG+6), with the intent of transferring 5 days after ovulation. If LH was < 20 mIU/mL, FET was performed 7 days after trigger (hCG+7). Primary outcomes included clinical pregnancy and live birth rates. To account for correlation between cycles, a generalized estimating equation (GEE) method for multivariable logistic regression was used.
Results
Four hundred fifty-three mNC-FET cycles met inclusion criteria, of which 205 were in the LH/HCG+6 group and 248 were in the HCG+7 group. The overall clinical pregnancy rate was 64% and clinical miscarriage rate was 4.8%, with similar rates between the two groups. The overall live birth rate was 60.9% (61.0% in LH/HCG+6 group and 60.9% in HCG+7 group). After implementing GEE, the odds of CP (aOR 0.97, 95% CI [0.65–1.45], p = 0.88) and LB (aOR 0.98, 95% CI [0.67–1.45], p = 0.93) were similar in both groups.
Conclusions
In our study cohort, mNC-FET based on LH/HCG+6 versus HCG+7 had similar pregnancy outcomes.
Keywords: Luteinizing hormone, Natural cycle, Frozen embryo transfer
Introduction
A noticeable trend favoring frozen over fresh embryo transfer has been observed recently in the USA. There has been an annual increase with the most recent Society for Assisted Reproductive Technology (SART) data from 2016 showing an equal number of fresh compared to frozen embryo transfers from non-donor eggs [1–3]. Given changes in the current practice pattern favoring frozen embryo transfer, rates are likely to be significantly higher over the last 4 years.
There are some potential benefits of medicated FET protocols for both IVF programs and patients, such as the ability to control timing, avoid cancelations due to delayed ovulation or anovulation, and extend duration of estradiol exposure if endometrial development is suboptimal. However, an mNC-FET may offer advantages to the patient that justify its use, such as avoiding the need for estradiol and progesterone administration as well as potential benefits to maternal and fetal health outcomes [4]. The majority of cycles performed in our center are mNC-FET.
While common sense dictates that the transfer should be performed at the time of highest endometrial receptivity, there is no consensus on the optimal timing of mNC-FET [5]. Prior studies have suggested mixed evidence as to the utility of timing frozen embryo transfer based on the presence of LH surge. In one randomized controlled trial of 124 patients undergoing mNC-FET using day 3 embryos, the authors found that spontaneous LH surge, defined as > 180% increase in LH from prior check, was associated with higher pregnancy rates. In this study, embryo transfer was planned 5 days after spontaneous LH surge or 5 days after hCG trigger if no spontaneous LH surge occurred; therefore, no adjustments in FET timing took place [6]. In another prospective, non-randomized trial of 233 patients undergoing mNC-FET using day 4 embryos, the authors found that spontaneous LH surge did not affect pregnancy rates. All patients were triggered after the dominant follicle reached > 17 mm with an appropriate endometrium. The presence of LH surge was defined as > 10 IU/l, but again did not alter FET timing. The authors concluded that performing a single LH determination before hCG administration in ultrasound-monitored mNC-FET provided no additional clinical value [7]. Of note, these studies included day 3 and day 4 embryos with no confirmation of euploidy and in smaller cohorts than our current study. In a more recent retrospective study of mNC-FET from 2017, the authors found higher pregnancy rates in cycles that transferred non-biopsied blastocysts after LH surge but similar pregnancy rates in 284 cycles that transferred PGT euploid blastocysts regardless of LH surge [8].
Given the findings suggested in the current literature, more data is needed to shed light on the impact of adjusting timing of mNC-FET based on the presence of LH surge, specifically for euploid blastocysts. Our practice is to adjust the timing of FET based on LH ≥ 20 mIU/mL. In the current study, we compare pregnancy outcomes of mNC-FET in women who had spontaneous LH surge and transferred 6 days later compared to women without detected LH surge on the day of hCG trigger and transferred 7 days after trigger.
Materials and methods
Patient selection
This retrospective cohort analysis included all patients who underwent mNC-FET with autologous, vitrified euploid blastocysts at a single academic institution starting from May 1, 2016, when we relocated to a new clinic and laboratory facility, to March 30, 2019. Prior workup included confirmation of a normal uterine cavity, including hysteroscopy, hysterosalpingogram, or saline infusion sonogram. Decision to proceed with mNC-FET was made based on the patient’s history of regular menstrual cycles as well as provider and patient preference. While some programs require the embryo quality to be a grade of BB or better prior to biopsy, our clinic policy is to biopsy embryos with a grade of CC or better. IRB approval was obtained.
mNC-FET protocol
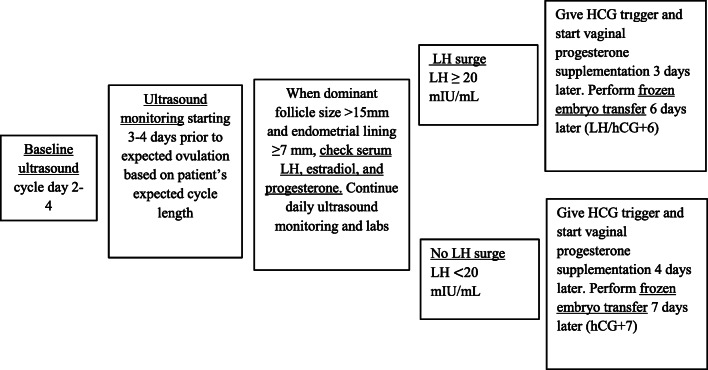
The standard protocol for mNC-FET began with a baseline ultrasound on cycle day 2–4 (Fig. 1). Ultrasound monitoring was initiated 3–4 days prior to expected ovulation based on each patient’s expected cycle length. When the dominant follicle was > 15 mm, serum LH, estradiol, and progesterone were checked. Ultrasound monitoring and lab testing were continued daily until the dominant follicle was ≥ 18 mm or positive LH surge was noted. A positive LH surge was defined as LH ≥ 20 mIU/mL using the Elecsys Assay (intra-assay coefficient of variability < 1.8%, inter-assay coefficient of variability < 5.2%). If LH was ≥ 20 mIU/mL, hCG trigger was given for reinforcement after confirming the LH surge, and FET was performed 6 days later (LH/hCG+6), with the intent of transferring a thawed blastocyst 5 days after ovulation. If LH was < 20 mIU/mL, hCG trigger was given that evening and FET was performed 7 days later (hCG+7). The trigger consisted of 250 mcg recombinant hCG (Ovidrel, EMD Serono). Ideally, patients underwent mNC-FET after achieving an endometrial thickness of ≥ 7 mm. However, if their cycle history showed their maximum endometrial thickness to be lower than this threshold, but a personal best, exceptions were made for them to proceed. Vaginal progesterone supplementation (Endometrin 100 mg vaginally twice a day or Crinone 8% gel vaginally daily) was started 3 days after spontaneous LH surge or 4 days after hCG trigger. Serum βhCG was obtained 9 days after embryo transfer, and transvaginal ultrasound was performed to evaluate for viable intrauterine pregnancy at 6–7 weeks gestation.
Fig. 1.
mNC-FET protocol
Independent variables
Baseline demographics were collected including age at retrieval and at transfer, body mass index (BMI), smoking status, ethnicity, race, and parity. Cycle characteristics were also collected including hormone testing as detailed above and endometrial thickness.
Outcome measures
Our primary outcomes of interest were clinical pregnancy rate, defined as the presence of fetal cardiac activity on transvaginal ultrasound per total number of cycles, and live birth rate, defined as a live infant born after 24 weeks of gestation per total number of cycles. Our secondary outcomes of interest included incidence of positive βhCG, biochemical miscarriage, pregnancy of unknown location (PUL)/ectopic pregnancy, intrauterine pregnancy, and clinical miscarriage. We defined positive βhCG as a serum βhCG > 5 mIU/mL. We defined a biochemical miscarriage as a rise and fall in βhCG without evidence of a clinical pregnancy. We defined pregnancy of unknown location/ectopic pregnancy as a rising βhCG without evidence of intrauterine pregnancy. We defined intrauterine pregnancy as the presence of an intrauterine gestational sac on transvaginal ultrasound. We defined clinical miscarriage as the loss of a clinical pregnancy prior to 20 weeks gestational age. We hypothesized that there was no difference in mNC-FET pregnancy outcomes in women who had spontaneous LH surge and transferred 6 days later compared to women without detected LH surge on the day of hCG trigger and transferred 7 days after trigger.
Statistical analysis
We calculated descriptive statistics for baseline characteristics by patient and cycle-varying characteristics by cycle, stratified by cohort (LH/hCG+6 and hCG+7). Means and standard deviations are reported for continuous variables, and frequencies and percentages are reported for categorical variables. We compared patient and cycle characteristics between LH/hCG+6 and hCG+7 using absolute standardized differences (ASD), a measure of the difference in means or proportions between two groups expressed in units of standard deviations [9]. In order to account for correlation between cycles per patient, we implemented the generalized estimating equation (GEE) method for multivariable logistic regression to assess differences in clinical pregnancy and live birth rates between the two protocols while adjusting for age at transfer, baseline BMI, parity status at transfer, and baseline smoking status. We calculated adjusted odds ratios (aORs) and 95% confidence intervals (CIs) to evaluate the relative odds for clinical pregnancies and live births for the hCG+7 group versus the LH/hCG+6 group. All analyses were conducted with R version 3.6.2 and the library geepack was used for GEE analysis [10–13].
Results
A total of 386 patients underwent 520 mNC-FETs during the study period. Of these, 18 cycles in 11 patients were missing data regarding their LH value on the day of hCG trigger and were excluded. Forty-nine cycles were also excluded due to exogenous vaginal estradiol use due to suboptimal lining. Therefore, 347 patients who underwent 453 mNC-FETs were included in the final analysis. Of these, 205 (45%) transfers occurred 6 days after spontaneous LH surge reinforced with hCG trigger (LH/hCG+6) and 248 (55%) transfers occurred 7 days after hCG trigger alone (hCG+7). Of the 453 cycles, 442 (98%) involved single embryo transfers.
Of the total 347 patients, 177 (51%) were in the LH/hCG+6 group and 170 (49%) were in the hCG+7 group. Of the patients who underwent more than one cycle, 28 had a spontaneous LH surge in some transfer cycles and no spontaneous LH surge in others, which placed them in the LH/hCG+6 group for some and the hCG+7 group for others. The overall mean age of the entire cohort at retrieval was 35.9 years, and the mean age at first transfer was 36.5 years. The mean baseline BMI was 25.1 kg/m2. The majority of patients were never smokers (94.8%) and nulliparous (65.4%). Race was the only baseline demographic that was notably different between the two groups based on absolute standardized differences (Table 1).
Table 1.
Patient demographics
| Protocol | ||||
|---|---|---|---|---|
| Overall | LH/HCG+6 | hCG+7 | ASD* | |
| n (%) | 347 (100%) | 177 (51%) | 170 (49%) | |
| Age at first transfera | 36.5 (3.8) | 36.1 (3.8) | 36.9 (3.8) | 0.187 |
| Age at retrieval | 35.9 (3.8) | 35.5 (3.7) | 36.2 (3.9) | 0.187 |
| Number of transfers | 1.3 (0.6) | 1.3 (0.7) | 1.30 (0.6) | 0.109 |
| Nulliparous, n (%) | 227 (65.4) | 131 (74.0) | 96 (56.5) | 0.375 |
| BMI | 25.1 (5.2) | 25.2 (5.4 | 25.0 (5.1) | 0.040 |
| Non-smoker, n (%) | 329 (94.8) | 167 (94.4) | 162 (95.3) | 0.043 |
| Race, n (%) | 0.551 | |||
| South Asian | 88 (25.4) | 55 (31.1) | 33 (19.4) | |
| East Asian | 97 (28.0) | 59 (33.3) | 38 (22.4) | |
| White | 140 (40.3) | 49 (27.7) | 91 (53.5) | |
| Black or African American | 4 (1.2) | 3 (1.7) | 1 (0.6) | |
| Other | 16 (4.6) | 10 (5.6) | 6 (3.5) | |
| Unknown | 2 (0.6) | 1 (0.6) | 1 (0.6) | |
| Infertility diagnosis, n (%) | ||||
| Diminished ovarian reserve | 79 (22.8) | 33 (18.6) | 46 (27.1) | 0.201 |
| Ovulatory dysfunction | 14 (4.0) | 9 (5.1) | 5 (2.9) | 0.109 |
| Recurrent pregnancy loss | 47 (13.5) | 21 (11.9) | 26 (15.3) | 0.100 |
| Male factor | 87 (25.1) | 53 (29.9) | 34 (20.0) | 0.231 |
| Unexplained | 78 (22.5) | 40 (22.6) | 38 (22.4) | 0.006 |
| Endometriosis | 17 (4.9) | 9 (5.1) | 8 (4.7) | 0.018 |
| Uterine | 11 (3.2) | 8 (4.5) | 3 (1.8) | 0.158 |
| Tubal | 16 (4.6) | 7 (4.0) | 9 (5.3) | 0.064 |
| Single gene disorder | 20 (5.8) | 8 (4.5) | 12 (7.1) | 0.109 |
| Other | 19 (5.5) | 6 (3.4) | 13 (7.6) | 0.187 |
aMeans and standard deviations reported for continuous variables
*Absolute standardized differences calculated between LH/hCG+6 and hCG+7; this represents the difference in means or proportions between the two groups divided by the pooled standard deviation 0.2, 0.5, and 0.8 correspond to small, medium, and large differences respectively. Therefore, smaller standardized differences represent less difference between the two groups
**347 patients who underwent 453 mNC-FET were included in the final analysis. Of these, 149 patients only had LH/hCG+6 cycles, 170 patients only had hCG+7 cycles, and 28 patients underwent cycles with either LH/hCG+6 or hCG+7 transfers depending on the cycle. Therefore, patients who had ever experienced an LH/hCG+6 transfer were grouped together for Table 1 descriptive statistics comparisons
Endometrial thickness was similar between the two groups. Estradiol levels were moderately higher in the LH surge group, and, as expected, LH and progesterone levels were higher in the spontaneous LH surge group (Table 2).
Table 2.
Cycle characteristics on day of trigger
| Protocol | ||||
|---|---|---|---|---|
| Overall | LH/hCG+6 | hCG+7 | ASD* | |
| n (%) | 453 (100%) | 205 (45%) | 248 (55%) | |
| Endometrial thicknessa (mm) | 9.1 (1.5) | 9.3 (1.5) | 9.0 (1.4) | 0.191 |
| LH value (mIU/mL) | 26.8 (24.7) | 46.5 (25.1) | 10.8 (5.1) | 1.969 |
| Peak estradiol value | 257.9 (96.8) | 274.8 (94.2) | 244.4 (96.9) | 0.317 |
| Progesterone value | 0.5 (0.3) | 0.7 (0.3) | 0.3 (0.2) | 1.264 |
aMeans and standard deviations reported for continuous variables
*Absolute standardized differences calculated between LH/hCG+6 and hCG+7; this represents the difference in means or proportions between the two groups divided by the pooled standard deviation. 0.2, 0.5, and 0.8 correspond to small, medium, and large differences respectively. Therefore, smaller standardized differences represent less difference between the two groups
The overall positive βhCG rate was 72.4% and the intrauterine pregnancy rate was 65.8%, with similar rates between the LH/hCG+6 and hCG+7 groups. The overall clinical pregnancy rate was 64% and the overall live birth rate was 60.9%, with similar rates between the two groups. Of all cycles with a positive βhCG outcome, the overall biochemical miscarriage rate was 7.9% and pregnancy of unknown location/ectopic pregnancy rate was 1.2%. Of all cycles with a clinical pregnancy, the miscarriage rate was 4.8%. There were no significant differences in pregnancy outcomes between the two groups (Table 3).
Table 3.
Pregnancy outcomes
| Protocol | ||||
|---|---|---|---|---|
| Overall | LH/hCG+6 | hCG+7 | ASD* | |
| n (%) | 453 (100%) | 205 (45%) | 248 (55%) | |
| Positive βhCG, n (%) | 328 (72.4) | 151 (73.7) | 177 (71.4) | 0.051 |
| Biochemical miscarriage, n (%) | 26 (7.9) | 13 (8.6) | 13 (7.3) | 0.047 |
| PUL/ectopic pregnancy, n (%) | 4 (1.2) | 3 (2.0) | 1 (0.6) | 0.127 |
| Intrauterine pregnancy (+GS), n (%) | 298 (65.8) | 135 (65.9) | 163 (65.7) | 0.003 |
| Clinical pregnancy (+FCA), n (%) | 290 (64.0) | 131 (63.9) | 159 (64.1) | 0.004 |
| Clinical miscarriage, n (%) | 14 (4.8) | 6 (4.6) | 8 (5.0) | 0.021 |
| Live birth, n (%) | 276 (60.9) | 125 (61.0) | 151 (60.9) | 0.002 |
*Absolute standardized differences calculated between LH/hCG+6 and hCG+7; this represents the difference in means or proportions between the two groups divided by the pooled standard deviation. 0.2, 0.5, and 0.8 correspond to small, medium, and large differences respectively. Therefore, smaller standardized differences represent less difference between the two groups
After implementing GEE in a multivariable logistic regression adjusting for age at transfer, BMI at transfer, parity status at transfer, and baseline smoking status, the LH/hCG+6 group had a similar odds of clinical pregnancy (aOR 0.97, 95% CI [0.65–1.45], p value = 0.88) and live birth (aOR 0.98, 95% CI [0.67–1.45], p = 0.93) compared to the hCG+7 group.
Discussion
Our study aimed to better understand whether timing of mNC-FET based on a spontaneous LH surge has an impact on pregnancy and live birth rates. The point estimates for the ORs are indicative of no difference between clinical pregnancy and live birth rates between the two groups. This suggests that in the presence of a healthy estrogen-producing follicle and lining, providers can feel equally confident in proceeding with FET 7 days after hCG trigger in the absence of an LH surge or 6 days after spontaneous LH surge. The confidence intervals for both outcomes may warrant further research to confirm our findings.
Current review of the literature supports that mNC-FET is non-inferior to medicated FET. Mounce et al. conducted a pilot randomized controlled trial in 2015 which showed no statistically significant difference between implantation, pregnancy, and live birth rates between the natural and medicated FET arms [14]. Similarly, no differences were found by Cerrillo et al. in 2017 and Kalem et al. in 2018 during retrospective analyses of natural versus medicated FET protocol outcomes at their respective institutions [15, 16]. Prior review on this topic at our clinic, where the majority of FET are performed in modified natural cycles if patients have regular menses, also showed no difference between natural and medicated FET success rates [17].
Medicated FET protocols afford IVF clinics flexible timing given that the programming of the cycle with exogenous administration of estradiol and progesterone allows for convenient scheduling. Additionally, they provide cost savings to patients with less frequent ultrasounds and laboratory tests. The implementation of mNC-FET protocols requires more frequent ultrasounds and laboratory tests as well as less flexibility with scheduling due to close monitoring of spontaneous ovulation and lack of control over the day of transfer. However, avoiding hormone replacement which commonly includes intramuscular progesterone—reported to improve pregnancy rates in medicated FET cycles [18]—makes mNC-FET a more attractive option for patients. In addition, compared to medicated FET with an absent corpus luteum, mNC-FET has been recently associated with reduced chance of hypertensive disorders in pregnancy and maternal cardiovascular perturbations [19, 20]. Given these patient-centered benefits, more research similar to the current study focusing on clinical predictors in mNC-FET outcomes is needed.
In this study, we defined LH surge as ≥ 20 mIU/mL. For some patients, this value may represent an early phase of the surge and a repeat test on the following day could reveal a significantly higher LH value. Nonetheless, prior literature suggests a single determination of LH ≥ 20 mIU/mL is an appropriate representation of LH surge as it relates to the timing of the transfer. Using LH surge timing on all their patients in mNC-FET cycles, Bartels et al. found no difference in pregnancy rates when the transfer was performed 6 days from the day the LH reached ≥ 20 mIU/mL irrespective of the LH behavior on the following day [21].
Additionally, an increased portion of Asian patients comprised the LH/hCG+6 group and an increased portion of White patients comprised the hCG+7 group. Separate univariate analysis confirmed that race did not correlate with a difference in clinical pregnancy or live birth rates, thereby limiting its potential as a confounder. Race was not incorporated into the regression model for this reason.
The main strength of our study stems from the frequent use of mNC-FET in our program, which enabled a large sample size of 453 cycles. Additionally, as mentioned above, in contrast to some prior studies exploring the role of LH surge in NC-FET, all embryos transferred were euploid blastocysts, which reflects a common current practice model.
hCG has been reported to have luteal phase support benefits [22]. For this reason, an hCG trigger was uniformly administered to all patients regardless of LH surge status. In line with this rationale, a recent retrospective study by Reichman et al. reported a 12% increase in ongoing pregnancy rates for patients who received a booster hCG trigger within 1 day of LH surge in mNC-FET of euploid blastocysts [23].
Regarding choice of vaginal progesterone supplementation, Endometrin versus Crinone was decided primarily based on the insurance coverage of each patient. Prior studies in the literature showed no difference in pregnancy success rates between either form of vaginal preparation, including a meta-analysis in fresh embryo transfers and a randomized controlled trial in programmed frozen-thawed embryo transfers [24, 25]. Therefore, since it is not an issue in situations where luteal support is indicated, then it is less likely to be an issue in natural cycles with an intact corpus luteum and where progesterone supplementation is being added as a precaution in the event of an undiagnosed luteal phase defect.
There are certain limitations associated with our study. The mean age of patients was 36 years; however, the fact that this study included only euploid blastocysts mitigates this effect of relatively advanced maternal age. In addition, the majority of patients in this retrospective study were of Asian and Caucasian descent, which may limit generalizability to other ethnic groups.
Given the patient-centered benefits of limited exogenous hormone replacement and potential reduced risk of preeclampsia, mNC-FET remains an important and safe treatment option when feasible from an operational standpoint. Ultimately, our study supports that pregnancy and live birth rates do not appear to be affected when timing of mNC-FET is adjusted to LH/hCG+6 versus hCG+7 based on the presence of a spontaneous LH surge.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
J. K. Johal and B. Bavan are co first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention. American Society for Reproductive Medicine, Society for Assisted Reproductive Technology . Assisted reproductive technology national summary report. Atlanta: US Dept of Health and Human Services; 2014. p. 2016. [Google Scholar]
- 2.Centers for Disease Control and Prevention. American Society for Reproductive Medicine, Society for Assisted Reproductive Technology . Assisted reproductive technology national summary report. Atlanta: US Dept of Health and Human Services; 2015. p. 2017. [Google Scholar]
- 3.Centers for Disease Control and Prevention. American Society for Reproductive Medicine, Society for Assisted Reproductive Technology . Assisted reproductive technology national summary report. Atlanta: US Dept of Health and Human Services; 2016. p. 2018. [Google Scholar]
- 4.Baker VL, Iko I, Segars J. Is a frozen embryo transfer in a programmed cycle really the best option? J Assist Reprod Genet. 2019;36:935–937. doi: 10.1007/s10815-019-01449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabibzadeh S. Molecular control of the implantation window. Hum Reprod Update. 1998;4:465–471. doi: 10.1093/humupd/4.5.465. [DOI] [PubMed] [Google Scholar]
- 6.Fatemi HM, Kyrou D, Bourgain C, Van den Abbeel E, Griesinger G, Devroey P. Cryopreserved-thawed human embryo transfer: spontaneous natural cycle is superior to human chorionic gonadotropin-induced natural cycle. Fertil Steril. 2010;94:2054–2058. doi: 10.1016/j.fertnstert.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 7.Groenewoud ER, Kollen BJ, Macklon NS, Cohlen BJ. Spontaneous LH surges prior to HCG administration in unstimulated-cycle frozen-thawed embryo transfer do not influence pregnancy rates. Reprod BioMed Online. 2012;24:191–196. doi: 10.1016/j.rbmo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Irani M, Robles A, Gunnala V, Reichman D, Rosenwaks Z. Optimal parameters for determining the LH surge in natural cycle frozen-thawed embryo transfers. J Ovarian Res. 2017;10:70. doi: 10.1186/s13048-017-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Communications in Statistics-Simulation and Computation. 2009;38:1228–1234. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 10.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing 2019, Vienna, Austria. https://www.R-project.org/. Accessed 1 Aug 2020.
- 11.Højsgaard S, Halekoh U, Yan J. The R Package geepack for generalized estimating equations. J Stat Softw. 2006;15:1–11. [Google Scholar]
- 12.Yan J, Fine J. Estimating equations for association structures. Stat Med. 2004;23:859–880. doi: 10.1002/sim.1650. [DOI] [PubMed] [Google Scholar]
- 13.Yan J. Geepack: yet another package for generalized estimating equations. R News. 2002;2:12–14. [Google Scholar]
- 14.Mounce G, McVeigh E, Turner K, Child TJ. Randomized, controlled pilot trial of natural versus hormone replacement therapy cycles in frozen embryo replacement in vitro fertilization. Fertil Steril. 2015;104:915–920. doi: 10.1016/j.fertnstert.2015.07.1131. [DOI] [PubMed] [Google Scholar]
- 15.Cerrillo M, Herrero L, Guillén A, Mayoral M, García-Velasco JA. Impact of endometrial preparation protocols for frozen embryo transfer on live birth rates. Rambam Maimonides Med J. 2017;8. [DOI] [PMC free article] [PubMed]
- 16.Kalem Z, Kalem M, Bakırarar B, Kent E, Gurgan T. Natural cycle versus hormone replacement therapy cycle in frozen-thawed embryo transfer. Saudi Med J. 2018;39:1102–1108. doi: 10.15537/smj.2018.11.23299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lathi RB, Chi YY, Liu J, Saravanabavanandhan B, Hegde A, Baker VL. Frozen blastocyst embryo transfer using a supplemented natural cycle protocol has a similar live birth rate compared to a programmed cycle protocol. J Assist Reprod Genet. 2015;32:1057–1062. doi: 10.1007/s10815-015-0499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine K, Richter KS, Widra EA, McKeeby JL. Vitrified blastocyst transfer cycles with the use of only vaginal progesterone replacement with Endometrin have inferior ongoing pregnancy rates: results from the planned interim analysis of a three-arm randomized controlled noninferiority trial. Fertil Steril. 2018;109:266–275. doi: 10.1016/j.fertnstert.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: Increased risks in programmed cycles. Am J Obstet Gynecol. 2019;221:126.e1–126.e18. doi: 10.1016/j.ajog.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 20.von Versen-Höynck F, Schaub AM, Chi YY, Chiu KH, Liu J, Lingis M, Stan Williams R, Rhoton-Vlasak A, Nichols WW, Fleischmann RR, Zhang W, Winn VD, Segal MS, Conrad KP, Baker VL. Increased preeclampsia risk and reduced aortic compliance with in vitro fertilization cycles in the absence of a corpus luteum. Hypertension. 2019;73:640–649. doi: 10.1161/HYPERTENSIONAHA.118.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartels CB, Ditrio L, Grow DR, O'Sullivan DM, Benadiva CA, Engmann L, Nulsen JC. The window is wide: flexible timing for vitrified-warmed embryo transfer in natural cycles. Reprod BioMed Online. 2019;39:241–248. doi: 10.1016/j.rbmo.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 22.van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2015;2015:CD009154. doi: 10.1002/14651858.CD009154.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichman DE, Stewart CR, Rosenwaks Z. Natural frozen embryo transfer with hCG booster leads to improved cycle outcomes: a retrospective cohort study. J Assist Reprod Genet. 2020;37:1177–1182. doi: 10.1007/s10815-020-01740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polyzos NP, Messini CI, Papanikolaou EG, Mauri D, Tzioras S, Badawy A, Messinis IE. Vaginal progesterone gel for luteal phase support in IVF/ICSI cycles: a meta-analysis. Fertil Steril. 2010;94:2083–2087. doi: 10.1016/j.fertnstert.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 25.Shiba R, Kinutani M, Okano S, Kawano R, Kikkawa Y. Efficacy of four vaginal progesterones for luteal phase support in frozen-thawed embryo transfer cycles: a randomized clinical trial. Reprod Med Biol. 2020;19:42–49. doi: 10.1002/rmb2.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]