Abstract
Arts syndrome or phosphoribosyl-pyrophosphate-synthetase-1 (PRPS1) deficiency is caused by loss-of-function mutations in the PRPS1 gene (Xq22.3). PRPS1 is an initial and essential step for the synthesis of the nucleotides of purines, pyrimidines, and nicotinamide. Classically, affected males present with sensorineural hearing loss, optic atrophy, muscular hypotonia, developmental impairment, and recurrent severe respiratory infections early in life. Treatment of a 3-year old boy with S-adenosylmethionine (SAM) replenished erythrocyte purine nucleotides of adenosine and guanosine, while SAM and nicotinamide riboside co-therapy further improved his clinical phenotype as well as T-cell survival and function.
Abbreviations: phosphoribosyl-pyrophosphate-synthetase-1, PRPS1; 5-phosphoribosyl-1- pyrophosphate, PRPP; PRPP synthase, PRPPS; nicotinamide adenine dinucleotide, NAD; NAD phosphate, NADP; S-adenosylmethionine, SAM; Nicotinamide riboside, NR; Adenosine triphosphate, ATP; Guanosine triphosphate, GTP
Highlights
-
•
Improves t-cell survival and function in PRPS1 deficiency
-
•
Improves overall well-being
-
•
Results in less (severe) infections
-
•
Is well tolerated
1. Introduction
The synthesis of 5-phosphoribosyl-1- pyrophosphate (PRPP) is catalysed by PRPP synthase (PRPPS), an essential early step for the synthesis and salvage of purine nucleotides and the synthesis of pyrimidine nucleotides and the diphosphopyridine nucleotides nicotinamide adenine dinucleotide (NAD) and NAD phosphate (NADP). Arts syndrome (OMIM 301835, ORPHA:1187) is caused by loss-of-function mutations in the PRPS1 gene (Xq22.3), which encodes for the enzyme phosphoribosyl-pyrophosphate-synthetase-1 (PRPS1).
Affected males present with early-onset progressive hearing loss, peripheral neuropathy, severe muscular hypotonia, optic atrophy, ataxia, developmental impairment, and frequent severe respiratory infections [[1], [2], [3], [4], [5], [6]].
S-adenosylmethionine (SAM) is a PRPPS-independent source of purine nucleotide precursors and crosses the blood-brain barrier. Treatment with 30 mg/kg/d SAM reduced the number of respiratory tract infections, need for respiratory support and hospitalization days and stabilised ataxia and hearing impairment in two Australian brothers with Arts syndrome [3,5]. NAD and NADP are coenzymes in a number of essential cellular pathways, particularly oxido-reductive processes. Conversion of nicotinamide or nicotinic acid to NAD(P) is PRPP- dependent [7] and patients with a dysfunctional, superactive PRPS1 have extremely low NAD(P) concentrations in erythrocytes [8]. Nicotinamide riboside (NR) can form NAD(P) independent from PRPP, using NR kinases 1 and 2 to produce nicotinamide mononucleotide which is adenylated to NAD [9,10]. NR is contained in low concentrations in milk and dairy products, and freely available as a nutrition supplement. NR supplementation (250 and 500 mg) resulted in a dose-dependent, significant increase of NAD concentrations in whole blood without serious adverse events in 120 healthy 60 to 80-year-old probands [11].
2. Methods
Parents gave their informed written consent to a trial with SAM and NR co-therapy. SAM 200 mg capsules and NR 300 mg capsules were available as oral nutrition supplements. SAM was administered 2 × 200 mg (~28 mg/kg/d) [3,5] from month 39 of life. Based on safety studies in adults, NR was given from month 43 of life with 1x300mg per day [11].
2.1. Laboratory methods
Analysis of PRPP synthetase activity in erythrocytes was performed as described elsewhere [12]. For nucleotide analysis, dried erythrocyte pellets were dissolved and the total protein content was measured [13]. Nucleotide profiles were determined by ion-exchange HPLC [14]. NAD and NADP were identified by retention times and UV spectra (details in supplementary material).
T-cells were isolated from peripheral blood mononuclear cells. CD4 and CD8 T-cells were stimulated in the presence of 50 IU/ml (CD4 T-cells) or 25 IU/ml (CD8 T-cells) of IL-2. For the secondary response, CD8 T-cells were stimulated for approx. 40 h, harvested, and rested for 5 days. They were then re-stimulated. Cytokine secretion was quantified in the cell culture supernatants at approximately 40 h post-activation for the primary response, and after overnight activation for the secondary response. Cell numbers were enumerated after live/dead staining, proliferation was assessed using intracellular staining and flow cytometric analysis.
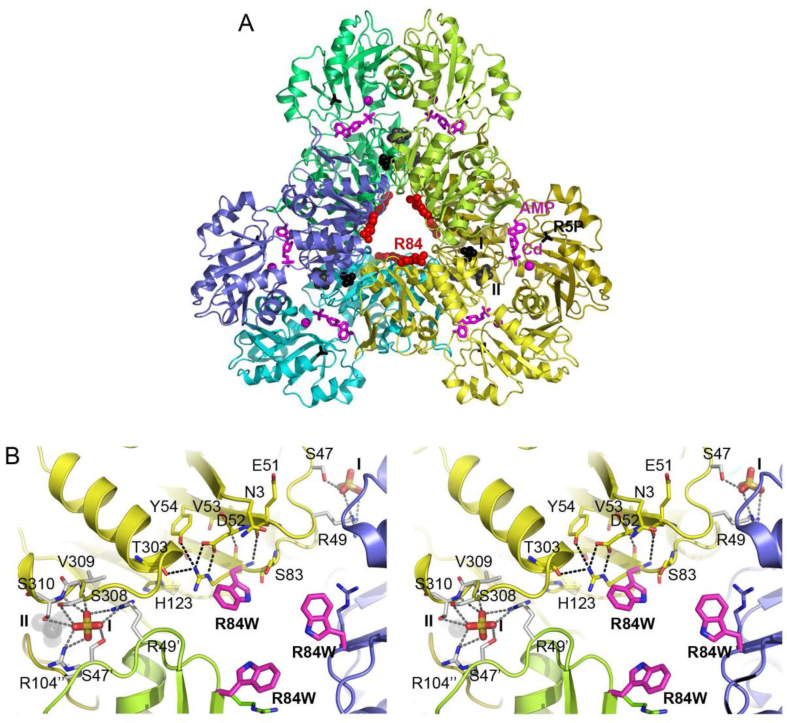
Mutation effect analysis and manual introduction of the altered amino acid side side chain in the crystal structure of human PRPS-1 from Li et al. [15] (PDB-Id: 2HCR) were performed with WinCoot [16]. Fig. 1 was generated with PyMol [17].
Fig. 1.
Three-dimensional structure of the PRPPS and environment of the p.Arg84Trp site. (A) The PRPPS hexamer as assembled from PDB entry 2HCR, which contains AMP (sticks in magenta) and a Cd2+ ion (magenta sphere) in the ATP binding site, and sulfate ions in the ribulose-5-phosphate (R5P, black sticks) binding site and allosteric sites I and II (space fill models in black and dark grey, respectively). Each monomer is coloured differently, but similar shades were used for those forming the more tightly interacting homodimers. A space-fill model of the Arg84 side chain in red indicates the variant site. For clarity, the ligands and variant site are labelled in one of the subunits only. (B) Close-up stereo views of the p.Arg84Trp substitution site. The side chain conformation causing the least steric clashes is chosen for the introduced Trp84 shown as stick model with carbon atoms in magenta. The replaced Arg84 side chain is shown with thinner sticks and carbon atom color corresponding to that of the respective subunit. Details of the hydrogen bonding interactions of Arg84 and of the sulfate ion bound at allosteric site I (labelled I) are shown for one of the subunits. Residues hydrogen bonding to Arg84 or sterically clashing with the variant-introduced Trp84 side chain are hereby depicted with carbon atoms in yellow, whereas sulfate ion-binding residues are shown with carbon atoms in white. The latter originate from three different subunits, as indicated by one, two or no apostrophes in the residue label. Hydrogen bonds are depicted as dashed lines. The nearby allosteric site II is labelled II and contains a sulfate ion shown as space fill model in dark grey.
3. Patient
The patient is the first son of healthy, non-consanguineous Swiss parents. In his first year he presented with congenital sensorineural deafness, recurrent prolonged severe respiratory infections, muscular weakness and hypotonia, pendular nystagmus, visual impairment and optic atrophy, mixed axonal demyelinating peripheral neuropathy, ataxia, bilateral pedes cavi, decreased tendon reflexes, regress of motor skills and developmental delay. Brain MRI (15 months) showed only slight myelinisation delay.
By 38 months, the boy had severe muscular hypotonia and ataxia, almost complete loss of head-control, he was unable to ambulate and spoke only ~10 single words (bilateral cochlear implants installed at 18 months). Binocular visual acuity was markedly reduced (Lea Panel: 0.009; small items located by touch) and the visual field was substantially restricted (~ 30° horizontal / ~10° vertical). CD4-, CD8-, T- and B-cell counts, immunoglobulins, subclasses and vaccination antibodies were normal. Percentage of memory and NK-cells were in the upper range of normal. NK-cells absolute count (1503/μl) slightly exceeded norms (100–1000/μl). Metabolic investigations including uric acid and urinary pyrimidine and purine measurements were normal.
At this time, a hemizygous missense variant in the PRPS1 gene (c.250C > T; p.Arg84Trp) of unknown clinical significance (American College of Medical Genetics classification criteria) was identified. Its disease-causing nature was proven by substantially decreased PRPP synthase activity in erythrocytes [0.1 nmol/min.mg protein; normal range (patients admitted to our hospital with clinical and biochemical findings not indicative of inborn errors in purine metabolism) 0.41–1.46 nmol/min.mg protein], the clinical phenotype and the confirmation of de novo occurrence after maternal segregation analysis.
Immediately from initiation of treatment, interval time between respiratory infections, which had been nearly inexistent, became longer (up to three months) and the boy recovered easier from infections according to parents' reports. Muscular strength, speech (active use of >30 words), ability to play, fatigue and overall well-being improved markedly. Treatment-related adverse events were not noted; homocysteine and methionine concentrations remained normal.
4. Laboratory workup
Human PRPPS is a homohexameric enzyme assembled from three tightly interacting dimers (Fig. 1A) to two sandwiched 3-rings [14]. It contains one catalytic and two allosteric sites per subunit. Each subunit consists of an N-terminal and a C-terminal domain, which both contribute to formation of the catalytic site. Allosteric site I, located at an interface between three subunits, can competitively bind the inhibitor adenosine diphosphate (ADP) or the activator inorganic phosphate. Allosteric site II is situated close to site I at the interface between monomers forming a homodimer. Phosphate binding to this site promotes binding of Mg-ATP to the catalytic site. The variant site Arg84 is located at the centre of the PRPPS hexamer (Fig. 1A), and plays an important role in connecting and maintaining the conformations of loops that carry residues contributing to effector binding at allosteric site I (Fig. 1B). Correspondingly, Arg84 is either conserved or conservatively exchanged to lysine or glutamine in bacterial to mammalian PRPPS. Replacement of Arg84 by tryptophan causes steric clashes with surrounding residues and inserts a hydrophobic side chain into a very polar environment. The Arg84Trp exchange is therefore prone to cause larger structural changes in this interface region between subunits that are very likely to have a negative impact on architecture of and ligand binding to allosteric site I. It may also lead to partial structural collapse, misfolding and/or improper hexamer assembly.
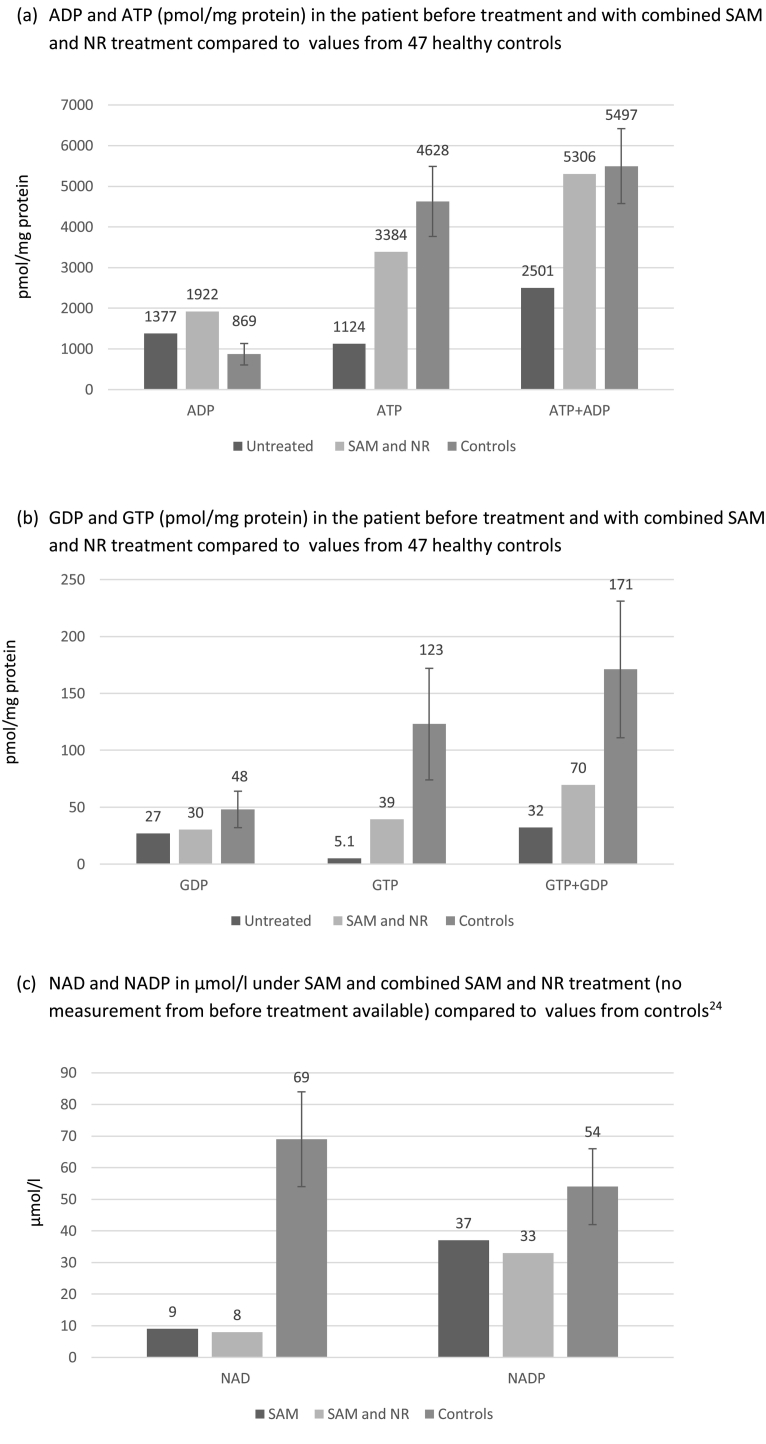
SAM treatment results in replenishment of the purine nucleotides adenosine triphosphate (ATP) and guanosine triphosphate (GTP) in erythrocytes (Fig. 2). NAD is virtually undetectable and NADP is very low in other PRPPS-deficient patients [3,8]. Especially for NADP, improvement can be seen with SAM and NR co-therapy, the pool gets replenished to the amount of 61% of control values. NAD, however, reaches only 12% of normal concentrations.
Fig. 2.
Nucleotide levels in erythrocytes in the patient under different treatment conditions and in healthy controls. Values of controls are given as means with standard deviations [24].
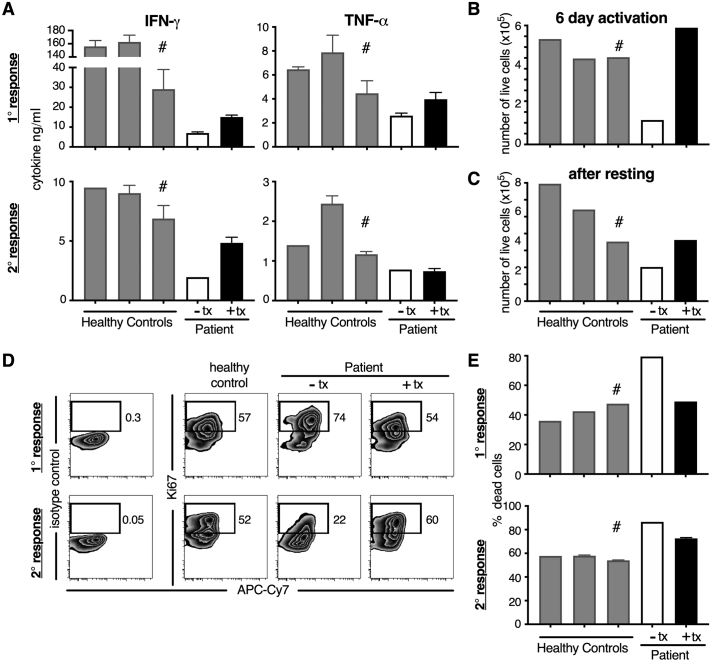
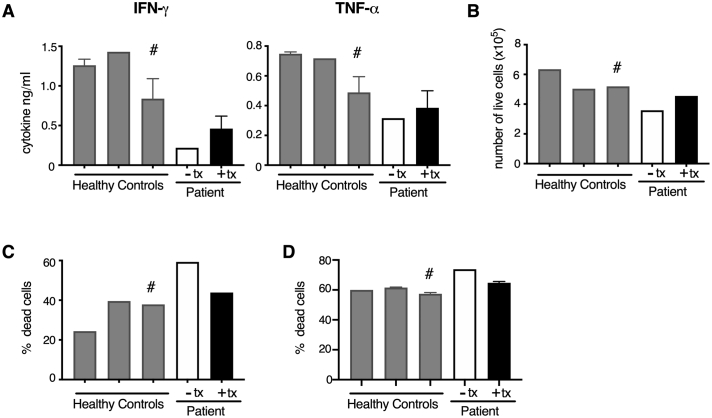
CD8 T-cell analyses were performed on patient samples collected after 24 h of treatment withdrawal (“off treatment”) and on SAM and NR co-therapy (“on treatment”). Upon activation “off treatment” CD8 T-cells had diminished inflammatory cytokine responses (IFN-ɣ and TNF-α, with IFN-ɣ more affected). Following primary and secondary stimulation, IFN-ɣ decreased 4 fold and 3.5 fold, and TNF-α decreased 1.7 fold and 1.5 fold, respectively, compared to the adolescent healthy control. The patient's “off treatment” CD8 T cells also had increased cellular death compared to healthy controls (Fig. 3). “On treatment”, CD8 T-cells had enhanced IFN-ɣ and TNF-α in the primary response compared to the “off treatment” cells. In particular, IFN-ɣ increased 2 fold and 2.5 fold during the primary and secondary responses, respectively, compared to the “off treatment cells” bringing cytokine levels to ~52% (primary response) and ~ 70% (secondary response) of the adolescent healthy control levels. Additionally, during primary stimulation “on treatment” cells had a 1.5 fold increase in their TNF-α response compared to the response from “off treatment” cells (bringing TNF-α cytokine levels to ~89% of the adolescent healthy control levels), however no enhancement was seen in the “on treatment” cells during the secondary response (Fig. 3). Treatment brought cell numbers and survival back into the range of healthy donors during the primary response. During the secondary response “on treatment” cells had decreased cell death compared to “off treatment” cells and their proliferation was rescued (Fig. 3). The patient's “off treatment” CD4 T-cells also had reduced IFN-ɣ responses after activation (~3.8 fold reduction compared to the adolescent healthy control), and the IFNg response was increased 2 fold in the “on treatment cells” compared to the “off treatment” cells (Fig. 4). Similar to CD8 T cells, there was increased cell death after activation in the “off treatment” CD4 T cells that was partially rescued in the “on treatment” cells (Fig. 4). Lastly, inflammatory cytokine responses were not enhanced by SAM treatment only (without NR treatment) in CD4 T-cells, but only with SAM and NR co-therapy (Fig. 5; CD8 T-cell data not collected).
Fig. 3.
The patient’s T cells have diminished cytokine responses and survival, which are partially or fully rescued by treatment with SAM + NR. CD8 T cells from three healthy controls (two adult and one adolescent (16 y.o. designated with #)) and from the patient after 24 hours of treatment washout (- tx) or during treatment (+ tx) were activated with CD3 + CD46 (CD46 co-stimulation is important for optimal human effector CD8 T cell responses). (A) IFN-ɣ and TNF-α secretion was assessed in the cell supernatants at ~40 hours post-activation (1° response) and after rest and re-stimulation overnight (2° response). At 6 days post-activation (B) and 5 days after rest (C) the number of live cells was enumerated. Proliferation was assessed by intracellular Ki67 expression (D) and the percentage of dead cells was calculated (E) after the 1° and 2° response using flow cytometric analysis.
Fig. 4.
The patient’s CD4 T cells have diminished cytokine responses and survival, which are partially rescued by treatment with SAM + NR. CD4 T cells from three healthy controls (two adult and one adolescent (16 y.o. designated with #)) and from the patient after 24 hours of treatment washout (- tx) or during treatment (+ tx) were activated with CD3 + CD46, a necessary co-stimulator for human Th1 responses (CD46 deficient patients do not mount Th1 responses). (A) IFN-g and TNF-a secretion was assessed in the cell supernatants after activation, rest and overnight re-stimulation. (B) At 6 days post-activation the number of live cells was enumerated. The percentage of dead cells was calculated after the (C) primary stimulation and (D) secondary stimulation (overnight re-stimulation) using flow cytometric analysis.
Fig. 5.
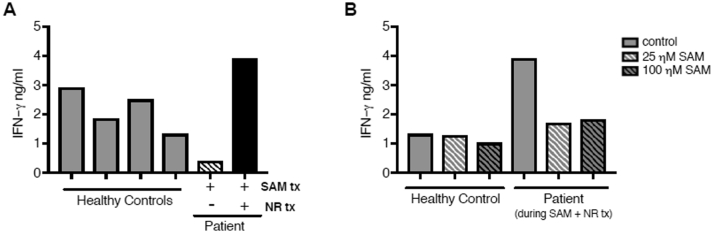
The patient's CD4 T cells have diminished IFN-γ cytokine responses, which are rescued by combined treatment with SAM + NR, but not with SAM alone. CD4 T cells from four healthy controls (naïve CD4 T cells from two adults and total CD4 T cells from a 16 and 17 year old) and the patient during SAM treatment only or SAM + NR treatment and activated with CD3 + CD46, a necessary co-stimulator for human Th1 responses (CD46 deficient patients do not mount Th1 responses). (A) IFN-γ secretion was assessed in the supernatants at ~40 h post-activation. (B) CD4 T cells from a healthy control and the patient during SAM + NR treatment were activated with CD3 + CD46 in the presence of vehicle control or 25 nM or 100 nM of SAM and IFN-γ secretion was assessed at ~40 h post-activation.
5. Discussion
SAM and NR co-therapy in a male with Arts syndrome resulted in fewer and less severe infections, improved muscular strength and overall well-being. This reduced strain on the patient and his family despite the persisting audio-visual impairment and ataxia.
Biosynthesis of the nucleotides ATP and GTP is PRPP-dependent and levels are low in Arts syndrome. The results here demonstrated that oral SAM improved erythrocyte ATP and GTP levels sustainably. NAD and NADP, which are critical coenzymes for cellular function, were probably greatly deficient in the patient's erythrocytes [3,8]. These two critical nicotinamide nucleotides did not attain normal concentrations in erythrocytes during patient treatment with 300 mg oral NR, but NADP approached normal values. Unfortunately, NAD/NADP measurements could not be repeated with 400 mg/d oral NR. NADP is particularly important for microbiocidal attack through generation of reactive oxygen species by NADPH oxidase in neutrophils and other immune cells [3].
T-cell function and cytokine patterns were investigated because recurrent, severe respiratory infections are characteristic for Arts syndrome and T-cells play a critical role in the control of respiratory viral infections [18,19]. Purine nucleotides and NAD are important mediators of cell proliferation and survival and influence cell metabolism, e.g. by impacting mTOR activation (purines) [20,21] and glycolysis (NAD) [22,23]. Thus, the loss or dysregulation of these important metabolites in T-cells likely impairs cellular metabolism, resulting in defective effector responses. On treatment with NR and SAM, CD8 T-cell numbers and survival were partially or fully restored and the cells developed typical IFN-ɣ and TNF-α patterns. The data from the CD4 T-cell inflammatory cytokine responses indicate that this effect was not attributable to SAM treatment only, but required the addition of NR treatment.
In conclusion, the treatment response in this patient with Arts syndrome indicates that oral co-therapy with SAM to restore ATP and GTP levels and with NR to alleviate T-cell functional abnormalities, reduced and mitigated infections, improved muscular strength and overall well-being. Although complete replenishment of NAD and NADP pools in erythrocytes could not be achieved, SAM and NR co-therapy rescued the patient's T-cell responses. Further elucidation of the mechanism of T-cell dysfunction during PRPS1 deficiency, and how SAM and NR co-therapy restores T-cell responses is warranted.
One sentence take-home message (synopsis)
Males with PRPS1 deficieny (Arts syndrome) respond clinically and biochemically to co-treatment with oral S-adenosylmethionine and nicotinamide riboside.
Competing interest statement
Nina Lenherr, John Christodoulou, John Duley, Doreen Dobritzsch, Lynette Fairbanks, Alexandre Datta, Isabel Filges, Nicolas Gürtler, Jeroen Roelofsen, André B.P. van Kuilenburg, Gabor Szinnai, Claudia Kemper, Erin E. West and Martina Huemer have no financial or other interests related to the submitted work that could affect or have the perception of affecting the authors' objectivity, or could influence or have the perception of influencing the content of the article. No funding was secured for this study.
Details of ethical approval
The usage of patient data in this study was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Patient data used in this study were all assessed based on informed consent. The manuscript is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals.
Details of the contributions of individual authors
Prof Huemer and Dr. Szinnai conceptualized and designed the study together with Prof Duley and Prof Christodoulou, and reviewed and revised the initial manuscript drafted by Dr. Lehnherr. Drs Dobritzsch, Roelofsen, van Kuilenburg, West, Fairbanks and Prof Kemper contributed important laboratory work and drafted the respective parts of the manuscript (methods and results). Drs Datta, Gürtler and Filges collected genetic and neuropediatric data and critically reviewed the manuscript for this content.
All authors reviewed and revised the manuscript and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Acknowledgements
We thank our patient and his parents for their cooperation and support. We are grateful for clinical support from our colleagues Sarabel Frey, Anja Palmowski-Wolfe, Benno Röthlisberger, Daniel Trachsel and Andreas Wörner and we thank Gunhild Unterstab and Christoph Hess for their help with sample preparation. The research conducted at the Murdoch Children's Research Institute was supported by the Victorian Government's Operational Infrastructure Support Program.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2021.100709.
Appendix A. Supplementary data
Supplementary material
References
- 1.Mittal R., Patel K., Mittal J. Association of PRPS1 Mutations with Disease Phenotypes. Dis. Markers. 2015 doi: 10.1155/2015/127013. 2015:127013. Epub 2015 May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arts W.F., Loonen M.C., Sengers R.C. X-linked ataxia, weakness, deafness, and loss of vision in early childhood with a fatal course. Ann. Neurol. 1993;33:535–539. doi: 10.1002/ana.410330519. [DOI] [PubMed] [Google Scholar]
- 3.de Brouwer A.P., van Bokhoven H., Nabuurs S.B. PRPS1 mutations: four distinct syndromes and potential treatment. Am. J. Hum. Genet. 2010;9;86(4):506–518. doi: 10.1016/j.ajhg.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Synofzik M., Müller Vom Hagen J., Haack T.B. X-linked Charcot-Marie-Tooth disease, Arts syndrome, and prelingual non-syndromic deafness form a disease continuum: evidence from a family with a novel PRPS1 mutation. Orphanet J Rare Dis. 2014 Feb 14;9:24. doi: 10.1186/1750-1172-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X.Z., Xie D., Yuan H.J. Hearing loss and PRPS1 mutations: wide spectrum of phenotypes and potential therapy. Int. J. Audiol. 2013;52(1):23–28. doi: 10.3109/14992027.2012.736032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balasubramaniam S., Duley J.A., Christodoulou J. Inborn errors of purine metabolism: clinical update and therapies. J. Inherit. Metab. Dis. 2014;37:669–686. doi: 10.1007/s10545-014-9731-6. [DOI] [PubMed] [Google Scholar]
- 7.Ying W.N.A.D. +/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 2008 Feb;10(2):179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 8.Simmonds H.A., Fairbanks L.D., Morris G.S., Webster D.R., Harley E.H. Altered erythrocyte nucleotide patterns are characteristic of inherited disorders of purine or pyrimidine metabolism. Clin. Chim. Acta. 1988 Feb 15;171(2–3):197–210. doi: 10.1016/0009-8981(88)90145-3. [DOI] [PubMed] [Google Scholar]
- 9.Chi Y., Sauve A. Nicotinamide riboside, a trace nutrient in foods, is a vitamin B3 with effects on energy metabolism and neuroprotection. Curr Opin Clin Nutr Metab Care. 2013 Nov;16(6):657–661. doi: 10.1097/MCO.0b013e32836510c0. [DOI] [PubMed] [Google Scholar]
- 10.Rajman L., Chwalek K., Sinclair D.A. Therapeutic potential of NAD-boosting molecules: the in vivo evidence. Cell Metab. 2018;27(3):529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dellinger R.W., Santos S.R., Morris M. Repeat dose NRPT (nicotinamide riboside and pterostilbene) increases NAD+ levels in humans safely and sustainably: a randomized, double-blind, placebo-controlled study. NPJ Aging Mech Dis. 2017;3:17. doi: 10.1038/s41514-017-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porrmann J, Betcheva-Krajcir E, Di Donato N, Kahlert AK, Schallner J, Rump A, Schröck E, Dobritzsch D, Roelofsen J, van Kuilenburg ABP, Tzschach A. Novel PRPS1gain-of-function mutation in a patient with congenital hyperuricemia and facial anomalies. Am. J. Med. Genet. A 2017 173(10):2736–2742. [DOI] [PubMed]
- 13.Smith P.K., Krohn R.I., Hermanson G.T. Measurement of protein using Bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 14.Bierau J., Van Gennip A.H., Helleman J. The cytostatic- and differentiation-inducing effects of cyclopentenyl cytosine on human neuroblastoma cell lines. Biochem. Pharmacol. 2001;62:1099–1105. doi: 10.1016/s0006-2952(01)00756-0. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Lu Y., Peng B. Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site. Biochem. J. 2007;401:39–47. doi: 10.1042/BJ20061066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emsley P., Lohkamp B., Scott W. Features and development of coot. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLano W.L. DeLano Scientific; Palo Alto, CA, USA: 2002. The PyMOL Molecular Graphics System.http://www.pymol.org [Google Scholar]
- 18.Schmidt M.E., Varga S.M. The CD8 T cell response to respiratory virus infections. Front. Immunol. 2018; Apr 9;9:678. doi: 10.3389/fimmu.2018.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teijaro J.R., Verhoeven D., Page C.A. Memory CD4 T cell direct protective responses to influenza virus in the lungs through helper-independent mechanisms. J. Virol. 2010;84(18):9217–9226. doi: 10.1128/JVI.01069-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin J., Ren W., Huang X. potential mechanisms connecting purine metabolism and Cancer therapy. Front. Immunol. 30 July 2018;9 doi: 10.3389/fimmu.2018.01697. 1697 PMID: 30105018; PMCID: PMC6077182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoxhaj G., Hughes-Hallett J., Timson R. The mTORC1 signaling network senses changes in cellular purine nucleotide levels. Cell Rep. 2017 Oct 31;21(5):1331–1346. doi: 10.1016/j.celrep.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei J., Raynor J., Nguyen T.L., Chi H. Nutrient and metabolic sensing in T cell responses. Front. Immunol. 2017;8:247. doi: 10.3389/fimmu.2017.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulf H.B., Quinn W.J., Jiao J. Immunosuppression reverse engineered from cancer: Reducing nicotinamide adenine dinucleotide (NAD) impairs effector T cell function through glycolysis. J. Immunol. May 1, 2018;200(1 Supplement) 55.27. Abstract. [Google Scholar]
- 24.Simmonds H.A., Duley J.A., Davies P.M. Analysis of purines and pyrimidines in blood, urine and other physiological fluids. In: Hommes F., editor. Techniques in Diagnostic Human Biochemical Genetics: A Laboratory Manual. Wiley-Liss; New York, NY: 1991. pp. 397–424. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material