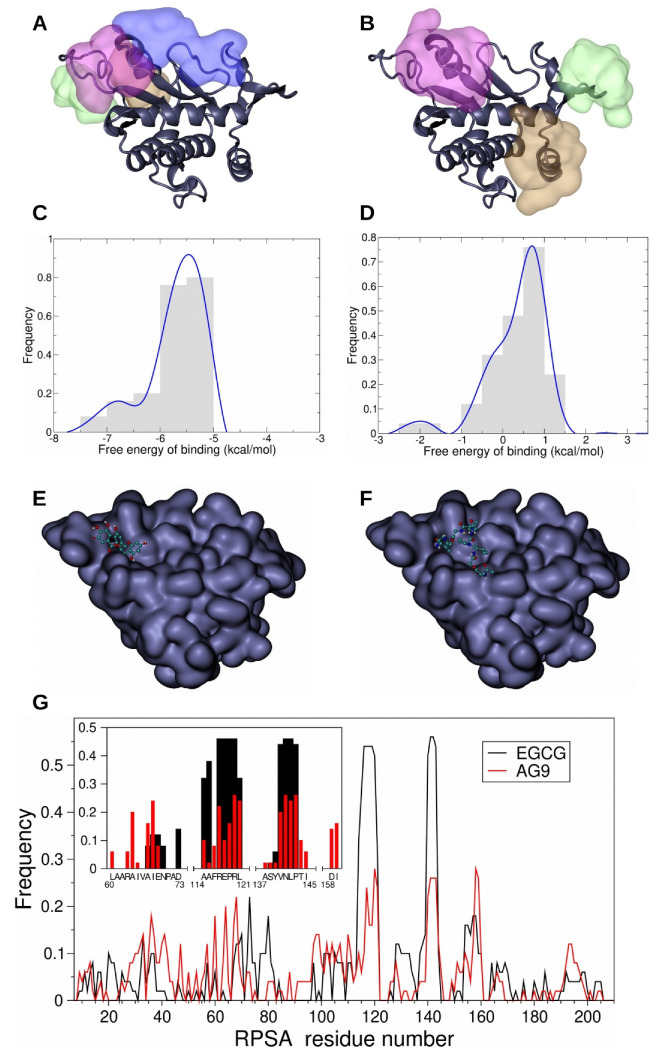
Figure 4.
Docking experiments of green tea-derived polyphenol EGCG and AG-9 peptide onto RPSA were performed using Autodock software and evidenced the existence of 4 preferred area of interaction (PAI) for EGCG (A) and 3 PAI for AG-9 (B). The color code used for the representation of PAI is linked to their population: the most populated one is represented in pink, the second in green, the third in brown, and the fourth in blue. Comparison of the 50 best results of EGCG and AG-9 docking experiments displays an overlapping area (pink PAI). Frequence distribution diagrams of the free energy of binding of the 50 best poses of EGCG onto RPSA demonstrated that those free energies of binding are comprised between −7.75 and −4.75 kcal/mol. The most frequent poses have a free energy of binding of −5.5 kcal/mol (C). For AG-9 peptide, the free energy of binding of the 50 best results of docking onto RPSA is comprised between −2.75 and +1.75 kcal/mol and the energy of binding of the most frequent position of AG-9 onto RPSA is around 0.75 kcal/mol (D). The best docking poses of EGCG (E) and AG-9 (F) are located on the same area at the surface of RPSA. Comparison of the frequency of contacts made by RPSA residues with the 50 best results of EGCG (black line) and AG-9 (red line) reveals two favored regions (frequency above the threshold of 0.2) of the protein (G). A focus on the poses associated with the first cluster of EGCG and AG-9 (G/close-up) allows the identification of the following hot spots (frequency above the threshold of 0.2 for the two ligands): R117, 120RL121, and the sequence 140VNLP143.