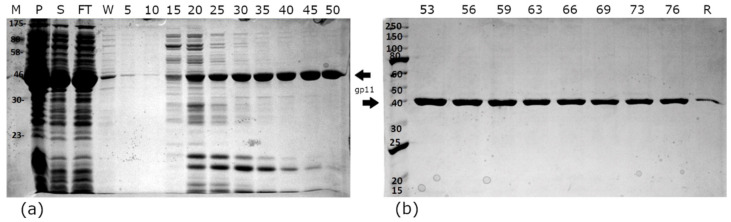
Figure 1.
Metal-affinity purification of the gp11 protein from cell lysate. SDS-PAGE gels show the early- (a) and late- (b) eluting fractions. Although the late-eluting fractions appeared to contain a single species, size-exclusion chromatography was used as a second purification step to increase the purity. Column labels: M—molecular weight standard; P—pellet (insoluble material from cell lysis); S—soluble material from cell lysis; FT—flow through from Talon resin binding, showing a protein that did not bind to the resin; W—10 mL wash of resin. Numbering references the fractions eluted from the column: 5 µL of cell lysate fractions (P, S, FT) and 10 µL of each fraction was loaded in each lane; R—residual protein remaining bound to the Talon resin, determined by mixing SDS dye with 10 µL of suspended resin.