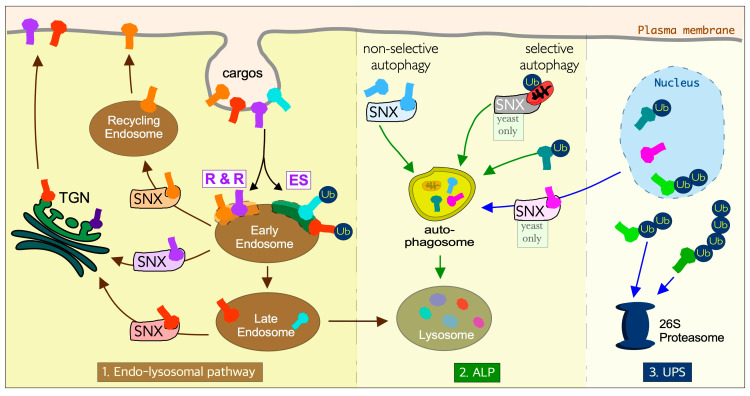
Figure 1.
Sorting nexins and ubiquitin coordinate the three distinct but interconnected protein proteolysis pathways. In the endo-lysosomal pathway, membrane proteins are sorted at the early endosome. Cargos destined for lysosomal degradation are marked by ubiquitination and internalized using endosomal sorting complexes required for transport pathways (ES). Cargos destined for recycling are retrieved either by the retromer or retrieval complex (R & R) coupled with various subclasses of sorting nexins (SNX- see text for details). In the autophagy-lysosome pathway (ALP) cargos are sequestered to the vacuole by double-membraned vesicles called autophagosomes by selective or non-selective mechanisms. Selective pathways in yeast are mediated by SNX-Bar heterodimers. In mammalian cells, the recognition of selective autophagy cargos is dependent upon ubiquitination (Ub). The ubiquitin-proteasomal system (UPS) targets short-lived regulatory proteins that are selectively targeted and degraded. TGN- trans-Golgi network, SNX-sorting nexin. In cells, the TGN and nucleus are in close proximity, whereas here they are drawn apart for clarity.