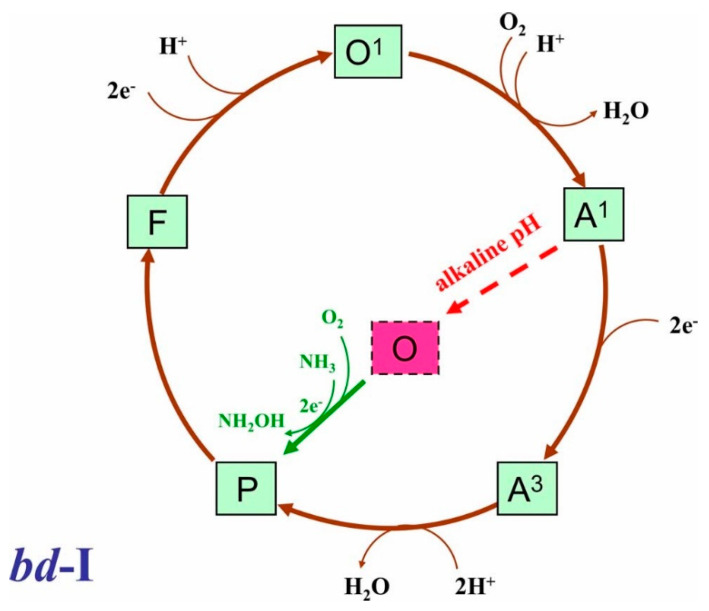
Figure 6.
The possible effect of ammonia on the catalytic cycle of cytochrome bd-I. Enzyme catalytic intermediates O1 (b5582+ b5953+ d3+–OH), A1 (b5583+ b5953+ d2+–O2), A3 (b5582+ b5952+ d2+–O2), P (b5582+ b5953+ d*4+=O2 where d*4+=O2 is a ferryl porphyrin π-cation radical [47,49]), and F (b5583+ b5953+ d4+=O2-) are shown. At alkaline pH, A1 is probably converted into the fully oxidized form O. O is not involved in the catalytic cycle [72]. NH3 possibly promotes the formation of P from O, thereby leading to the acceleration of the oxidase activity. It is also possible that NH3 reacts with O1 producing F. In the latter two reactions, NH3 serves as a two-electron donor being oxidized to NH2OH. The reaction of NH3 with O1 is not shown for the sake of simplicity.