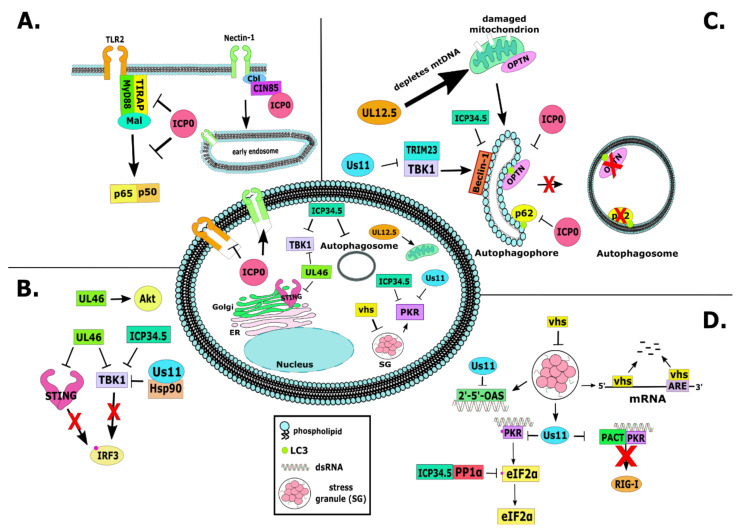
Figure 2.
Cytoplasmic functions of some non-essential HSV-1 proteins. (A): ICP0 participates in two major functions in the cytoplasm. First, ICP0 degrades the TLR2 adaptors TIRAP and Mal, thus blocking NF-κB activity. ICP0 also binds to the endocytosis adaptor CIN85 and along with Cbl promotes internalization of the viral entry receptor Nectin-1. This is a mechanism to promote progeny virus spread to uninfected cells. (B): The tegument protein UL46 blocks STING and TBK1, which prevents stimulation of interferon-regulated genes. ICP34.5 and US11 are also involved in blocking TBK1, emphasizing the importance of blocking TBK1 activity during HSV-1 infection. (C): The autophagy pathway is blocked during HSV-1 infection through binding of ICP34.5 to Beclin-1, thus preventing maturation of the autophagophore to an autophagosome. ICP0 has also been found to cause downregulation of p62 and OPTN proteins during infection, which may also serve as another mechanism of blocking selective autophagy. It has also been found that the protein encoded by UL12.5 causes depletion of mtDNA during infection, which causes damage to mitochondria. (D): HSV-1 prevents host translational shutoff from occurring during infection. One mechanism is through ICP34.5 binding to both PP1a and eIF2α, causing dephosphorylation of eIF2α and preventing shutoff of translation. HSV-1 also encodes vhs, which is a viral RNase that degrades AU-rich element (ARE) containing mRNAs. It has also been shown that vhs prevents the formation of cytoplasmic stress granules (SGs) during infection, which contain dsRNA that would otherwise cause PKR activation. HSV-1 also encodes US11, which blocks PKR, thus blocking host translational shutoff and innate immunity activation, as well as blocking PKR and PACT-induced activation of RIG-I during infection.