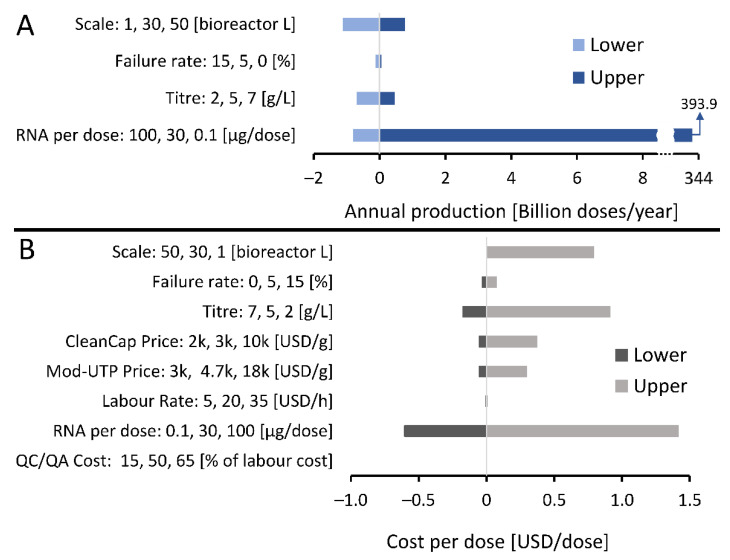
Figure 1.
Sensitivity analysis showing RNA vaccine drug substance manufacturing uncertainties and their impact on annual production amounts and costs per dose. Input variables which are uncertain, their uncertainty ranges and units are shown on the vertical y-axis and the outputs of drug substance annual production amounts and costs per dose and shown on the horizontal x-axis. The zero values shown on the horizontal x-axes and the corresponding vertical line indicates a baseline scenario which describes an RNA vaccine production process with a 30 L bioreactor working volume scale, a final titre in the bioreactor of 5 g/L, 44% combined losses in the downstream purification and formulation steps, 30 µg of RNA per vaccine dose, a production process failure rate of 5%, 5′ cap analogue (CleanCap) purchase price of 3000 USD/g, 1-methyl-pseudouridine (Mod-UTP) purchase price of 4700 USD/g, basic labour rate of 20 USD/hour, quality control testing (QC/QA) cost of 50% of the labour costs, and 444–471 production batches completed per year. (A) The impact of uncertainties and their ranges listed on the vertical y-axis on the amount of doses worth of lipid nanoparticles (LNP) formulated RNA that can be produced annually shown on the horizontal x-axis. Results are shown relative to a baseline scenario at zero on the x-axis, as described above. (B) The impact of uncertainties and their ranges listed on the vertical y-axis on the production cost of the LNP formulated RNA drug substance per dose shown on the horizontal x-axis. Results are shown relative to a baseline scenario at zero on the x-axis.