Abstract
Schizophrenia (SCZ) can be characterized as a basic self-disorder that is featured by abnormal temporal integration on phenomenological (experience) and psychological (information processing) levels. Temporal integration on the neuronal level can be measured by the brain’s intrinsic neural timescale using the autocorrelation window (ACW) and power-law exponent (PLE). Our goal was to relate intrinsic neural timescales (ACW, PLE), as a proxy of temporal integration on the neuronal level, to temporal integration related to self-disorder on psychological (Enfacement illusion task in electroencephalography) and phenomenological (Examination of Anomalous Self-Experience [EASE]) levels. SCZ participants exhibited prolonged ACW and higher PLE during the self-referential task (Enfacement illusion), but not during the non-self-referential task (auditory oddball). The degree of ACW/PLE change during task relative to rest was significantly reduced in self-referential task in SCZ. A moderation model showed that low and high ACW/PLE exerted differential impact on the relationship of self-disorder (EASE) and negative symptoms (PANSS). In sum, we demonstrate abnormal prolongation in intrinsic neural timescale during self-reference in SCZ including its relation to basic self-disorder and negative symptoms. Our results point to abnormal relation of self and temporal integration at the core of SCZ constituting a “common currency” of neuronal, psychological, and phenomenological levels.
Keywords: self-disorder, intrinsic neural timescale, auto-correlation window, power law exponent, EEG, rest-task modulation, enfacement illusion, EASE
Introduction
Temporal Integration I—“Common Currency” of Phenomenological, Psychological, and Neuronal Levels
The disturbance of the basic self (a structural instability of the first person-perspective) has been proposed to constitute a core trait-phenomenological feature of schizophrenia (SCZ).1–3 Basic self-disorder can be assessed with a well-established phenomenological instrument, ie, the EASE-scale (Examination of Anomalous Self-Experience).4 One key aspect of basic self-disorder is abnormal temporal integration (see below for definition) on a phenomenological level (time experience).5–8 Abnormal temporal integration is also described on the psychological (time perception) level6,9–15 as related to a discontinuous sense of self.2,5,8,16–18 The neuronal correlates of abnormal temporal integration as a key aspect of basic self-disorder remain unknown.
We understand temporal integration in a wide way, as connecting different time points by pooling and summing them (see also refs. 19,20). Connection between different time points can occur on different levels, phenomenological, psychological, and neuronal levels, thus providing their “common currency.” 21
On the phenomenological level, different time points are integrated into experience of time as it is manifest in the “stream of consciousness” 22–24 and “inner time consciousness.” 5–8 Psychologically, different stimuli at different time points are integrated into perception when we perceive a coherent scene or a melody composed of stimuli occurring at different time points; the distinction of psychological and phenomenological levels is relative and operational (rather than absolute and ontological) as both provide different methods to access related or even the same temporal integration. Finally, neural activities at different time points are also pooled, summed, and integrated, which can be measured by the brain’s intrinsic neural timescale.20
Temporal Integration II—The Brain’s Intrinsic Neural Timescale
Information processing in the brain is shaped by the spontaneous activity’s spatiotemporal dynamics constituting an intricate temporal structure. Such temporal structure is manifest in what has been described as “intrinsic neural timescale.” 25–28 The intrinsic neural timescale provides temporal windows within which different stimuli can be integrated and processed together (temporal integration on the psychological level).20,29 The intrinsic neural timescale can be investigated by calculating the autocorrelation window (ACW). The ACW measures repeating and correlating patterns in a signal, enabling us to test the relationship, eg, correlation in neural activity patterns across different points in time.25 It has been applied at cellular25,30,31 and system levels32–34 measuring temporal integration on the neuronal level.20,25–27,29
Longer ACW is related to slow frequencies while shorter ACW is modulated by stronger power in faster frequencies.29 The balance in power between slow and fast frequencies can be measured in frequency characteristics of periodic oscillations in electroencephalography (EEG) calculating power-law exponent (PLE) as an index if arrhythmic scale-free (“1/f noise”) brain activity.35–40 Like ACW in the time domain, the PLE can be conceived an index of temporal integration albeit in the frequency domain, ie, it measures the degree of integration in the power of different frequencies.26,27,29,38
Healthy subject studies in functional magnetic resonance imaging (fMRI)37 and EEG32 show that inter-individual variation in intrinsic neural timescales (ACW, PLE) explains a high degree of inter-individual variation in self-consciousness (see refs. 41–43). These data suggest that temporal integration on the neuronal level, ie, ACW and PLE, is related to temporal integration on psychological and phenomenological levels of self—this provides the background of our study.
Temporal Integration III—“Common Currency” of Neuronal, Psychological, and Phenomenological Changes of Self in SCZ
Several studies using fMRI44–47 or EEG9–13,48–53 observed changes during self-referential stimuli in SCZ; ie, reduced activity in cortical midline structures (medial prefrontal cortex and posterior cingulate cortex) (fMRI)44,46 and decreased amplitudes in event-related potentials.49–53 A multisensory self-recognition task (Enfacement illusion)54,55 found behavioral deficits in SCZ56,57 possibly related to deficits in temporal integration on the psychological level, the neuronal mechanisms of which remain unclear.
SCZ has been characterized by decreases in faster frequency power (gamma,58–60 alpha,61,62 and theta63–65). Given reduced fast frequency power in SCZ as well as the dependence of ACW and PLE on slow-fast frequency power balance, one would expect prolonged ACW and elevated PLE in SCZ on the neuronal level. Moreover, findings in healthy subjects (above) suggest that altered ACW and PLE on the neuronal level could be related to changes in temporal integration of self on psychological and phenomenological levels.
Aims, Hypotheses, and Experimental Design
The goal of our study is to investigate the brain’s temporal integration on the neuronal level by probing intrinsic neural timescale for the first time in SCZ (see33,66 for autism) including its relation to psychological and phenomenological changes of self. We hypothesized that temporal integration is abnormal in the self of SCZ providing the missing link or “common currency” 21 of phenomenological, psychological, and neuronal levels. To that end, we applied measures of temporal integration on all 3 levels: phenomenological, psychological, and neuronal.
Our first specific aim consisted in testing temporal integration on the neuronal level by measuring intrinsic neural timescales in EEG using ACW and PLE (as calculated over specifically the fractal rather than oscillatory component of the power spectrum; see Methods and Materials); ACW and PLE were measured during task states as well as in their relative difference to rest, ie, “rest-task modulation.” 40,67 Based on the findings on self-referential processing,9–13,48–53 we hypothesized that SCZ participants would show (1) prolonged ACW and abnormally high PLE and (2) reduced rest-task ACW/PLE difference during specifically the self-referential task (but not non-self-referential task).
Testing temporal integration on the psychological level during EEG, we applied a self-referential task (Enfacement illusion)54,55,57 and a non-self-referential task (auditory oddball paradigm)68–70: presenting morphed pictures of one’s own and another person’s face in a temporal sequence requires subjects to temporally integrate (on the psychological level) the different pictures’ time points under the self or, alternatively, segregate them when attributing them to the other person’s face, ie, non-self.
Our second specific aim was to investigate the relation of neuronal and psychological abnormalities of self to psychopathological and phenomenological changes, ie, the basic self-disorder.9–13,48–53 Applying a moderation model, we hypothesized that temporal integration on the neuronal level (rest-task modulation of ACW and PLE) during a task requiring temporal integration on the psychological level (Enfacement task) would moderate the impact of the phenomenological level (basic self-disorder) on psychopathological symptoms (like negative symptoms).
Methods and Materials
Subjects
Thirty-seven patients diagnosed with SCZ (mean age 22.11 years, 14 males) and 36 matched healthy controls (HC) (mean age 24.06 years, 14 males) were included in the study. The patients were recruited from 3 psychiatric outpatient clinics in Region Zealand in Denmark between May 2017 and February 2019. The majority of patients were under treatment with atypical antipsychotics in a stable post-acute symptomatic phase of their illness (see supplementary table S1; see supplementary material for inclusion and exclusion criteria).
Written consent was obtained from all participants after having received an oral and written description of the paradigms in the study. The study was approved by the regional data agency and ethics committee in Region Zealand, Denmark and in line with the ethical standards of the Declaration of Helsinki 2013.
Clinical Evaluation
The psychiatric evaluation was performed by K.E.S., a senior resident in psychiatry, who was reliability tested and trained by the founder of the EASE scale (J.P.) and a senior EASE expert (J.N.). All participants were evaluated with a comprehensive psychopathological examination, including Assessment of Positive and Negative Syndrome Scale (PANSS),71 the Operational Criteria Checklist for Psychotic Illness and Affective Illness (OPCRIT),72 the perceptual domain of the Bonn-scale (BSABS),73 and Examination of Anomalous Self Experience (EASE), that targets different domains of self-disorder (see supplementary material).4 A focused neurological examination was performed for vision, hearing, and sensation.
EEG—Rest and Task
Resting State
EEG was recorded with participants lying down with eyes open for 5 minutes. Participants were asked to fix their gaze at a particular point on the wall while relaxing. EEG was recorded continuously during the following paradigms:
Enfacement Illusion
The task consisted of 3 blocks of 4 facial recognition tasks. The tasks comprised pictures of the participants face and/or other faces, being dynamically morphed into each other on a screen. Each block had 4 morphing conditions: self-other, other-self, other1-other2, and other2-other1 (in randomized order). For further details, see ref. 56.
Oddball Paradigm
The paradigm consisted of 1000 tones at 1000 Hz, 60dB, presented with an inter-stimulus interval of 500 ms; 900 tones (90%) were standard tones of 50 ms duration and 100 (10%) were deviants of 100 ms duration. All tones were presented in a randomized order binaurally through headphones, while the participants watched a mute video on a screen.
EEG Acquisition and Analysis
Recording
The EEG recording system used was a Nicolet Nervus, version 5.94.1.534 with a V44 amplifying system and a sampling rate at 250 Hz. The Ag/AgCl electrodes were distributed with a WaveGuard cap-system comprising 20 electrodes applied according to the 10/20 system. Six additional low-row electrodes were used with eye-flick transducer, 2 ECG-electrodes, and a built-in NONN pulse oximetry.
Preprocessing
EEG preprocessing of the data was performed with Harvard Automated Preprocessing Pipeline for EEG (HAPPE).74 Data were filtered with a 1-Hz FIR highpass filter in EEGLAB.75 Because the Cleanline procedure traditionally used in the HAPPE pipeline failed to adequately suppress line noise in our data, we replaced this step with notch filtering at 50 and 100 Hz. Bad channels were rejected using HAPPE’s normalized log-power heuristic, and wavelet-thresholded ICA was performed to prepare data for regular ICA decomposition. Artifactual ICA components were rejected using MARA.76 Data were then segmented into 2-second non-overlapping epochs, and bad channels and segments were repaired using FASTER.77To preserve the continuity of time series for subsequent analyses, no segments were rejected at this step. Finally, data were re-referenced to a common average reference.
Calculation of ACW
The ACW was calculated according to the methods of Honey et al.29Autocorrelation was calculated using windows of 20-second length with an overlap of 50%. The maximum lag was set to 0.5 seconds, given that we have previously established that the ACW values agree for different maximum lag values (ranged from 0.1 to 1 seconds) (Wolff et al).32 The full-width-half-maximum of the main lobe of each of the autocorrelation functions was then computed for each epoch. ACW was estimated as the average of these values over all the epochs for each electrode and condition. ACW values represent the extent of the periodicity of the EEG signal, whereby longer ACWs can be interpreted as greater stability of the signal over time. The length of the ACW can be seen, therefore, as an index that summarizes the degree of regularity of a signal, with longer ACW associated with more a regular EEG signal.
Calculation of PLE
The PLE was calculated on the fractal (rather than oscillatory) component of the power spectrum (see supplementary material for details).
Calculation of Rest-Task Differences (ACW, PLE)
See supplementary material for details.
Statistical Analysis
Differences in ACW and PLE between SCZ and HC were assessed using cluster-based permutation testing (FDR-correction)78 implemented in the Fieldtrip toolbox.79 The Wilcoxon rank-sum statistic was used for channel-level statistics, and neighboring electrodes were determined using triangulation. For the moderation model80 testing for correlation of ACW/PLE with the interaction between psychopathological components (from principal component analysis [PCA]), the mean values of task-related ACW and PLE (for Enfacement illusion) all electrodes were used. For each PCA component and for each neural variable, we fit a linear model with the neural variable of interest plus its interactions with the other components as independent variables. For example, for component 1 and ACW, the model included terms for ACW plus its interactions with component 2, component 3, and the 3-way interaction.
Results
Phenomenological, Psychopathological, and Psychological Levels—Relation of EASE, PANSS, and Enfacement Illusion
The 5 domains of EASE as well as PANSS general, positive, and negative were as expected significantly higher in the SCZ group compared with HC (see supplementary table S2). In order to test for the relationship of self-disorder to positive and negative symptoms, we conducted PCA. The analysis of psychopathological symptoms yielded 3 components (table 1). The first component explained 48% of the variance and represented mainly the EASE variables, with contribution from the Bonn scale (self-component). The second component loaded strongly on the PANSS negative score, with contribution from PANSS general and negative correlation with EASE domain 5 (negative symptom component). The third component loaded strongly on PANSS positive symptoms, with some contribution from the EASE domain 5 and negative contribution from EASE domain 4 (positive symptom component).
Table 1.
Component Weights From the Principal Component Analysis of Psychopathological Scalesa
| Component 1 | Component 2 | Component 3 | |
|---|---|---|---|
| EASE domain 1 | 0.406283319 | −0.09740349 | −0.16122152 |
| EASE domain 2 | 0.435772802 | 0.058453723 | −0.05144734 |
| EASE domain 3 | 0.391909064 | 0.068597697 | −0.17367412 |
| EASE domain 4 | 0.317294974 | 0.026237335 | −0.46153488 |
| EASE domain 5 | 0.289602831 | 0.419820488 | 0.404926189 |
| Perceptual domain (Bonn) | 0.414047859 | 0.063385769 | 0.105968483 |
| PANSS Positive | 0.208911823 | 0.211443414 | 0.740784151 |
| PANSS Negative | 0.067811038 | 0.734997141 | 0.036286068 |
| PANSS General | 0.295328966 | 0.465301588 | −0.05395621 |
| Explained variance | 48.4% | 16.8% | 13.0% |
Note: EASE, Examination of Anomalous Self-Experience.
aSince all variables were z-scored before being entered into the analysis, the weights are directly comparable between scales. Variance explained by each component is included at the bottom of the table.
We found a weak but significant correlation between the total EASE score and self-recognition in the self-other direction (r = .25; P = .03) among all participants, supporting the relationship of the self-disorder on the phenomenological level to changes in self on the psychological level. Within the SCZ group separately, there was a nonsignificant weak negative correlation (r = −.25; P = .16).
Neuronal and Psychological Levels—Intrinsic Neural Timescale in Task and Task-Rest EEG
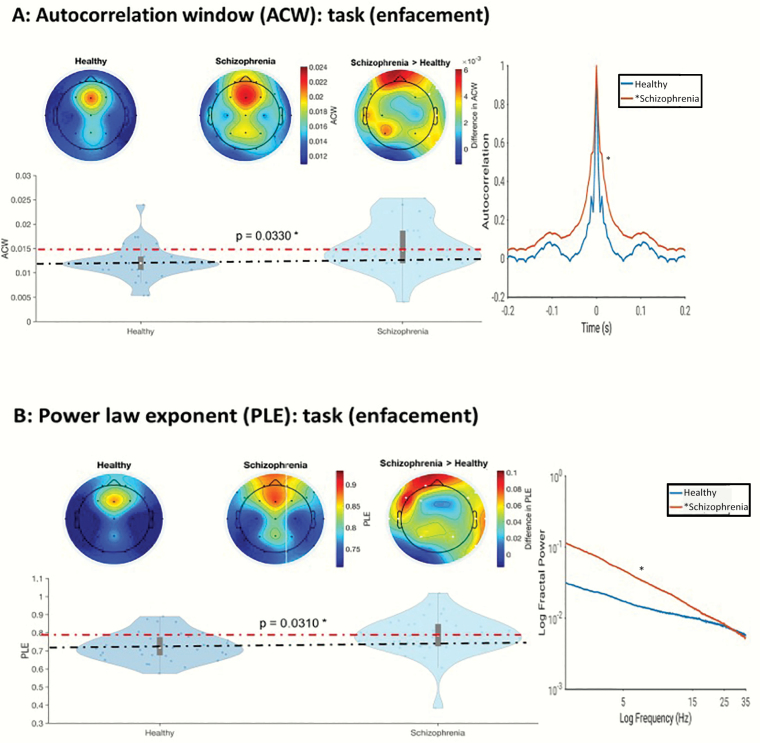
We observed significantly longer ACW in the Enfacement illusion task in SCZ compared with HC (P = .03) as obtained by cluster-based permutation that corresponds to FDR78,79 (which holds for all the following statistical values of ACW and PLE) (figure 1a). The PLE showed significant group difference during the Enfacement illusion: Schizophrenia (SCH) exhibited higher PLE than HC (P = .03) meaning that the slope was steeper as shifted toward slower frequencies in SCH (reflecting higher regularity with more long-range correlation in arrythmic fluctuations) (figure 1b). In contrast, no differences in ACW and PLE were obtained in the non-self-referential auditory oddball task (see supplementary figure S1 and table S3.1).
Fig. 1.
(A) Altered autocorrelation window (ACW) and (B) power-law exponent (PLE) in enfacement task. Scalp topographies of the ACW and PLE values are shown on top, and violin plots of the average values are shown at the bottom. For each figure, white dots in the rightmost topoplot represent electrodes, which form part of the significant cluster (following the cluster-based permutation test described in the text). Black dots in this plot represent electrodes with significant results at the channel level, but which did not form part of the cluster. Violin plots show the average value of ACW or PLE over only the white electrodes. In each figure, insets on the very right show the extremes of individual subjects’ data—the schizophrenic participant shown has the highest mean ACW or PLE over the white electrodes, and the healthy participant shown has the lowest mean ACW or PLE.
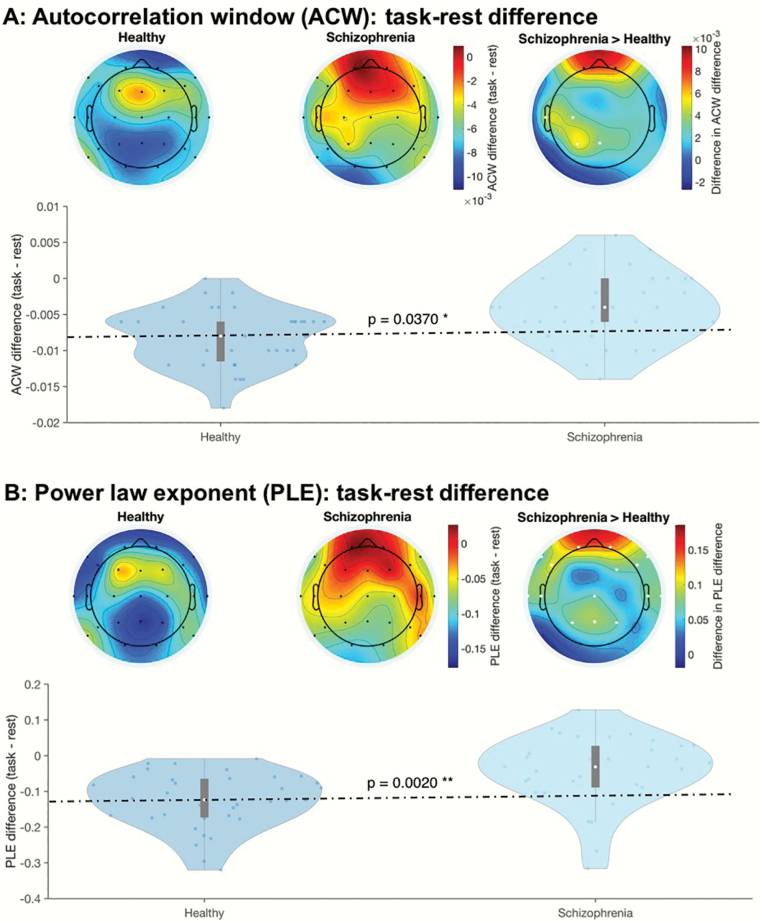
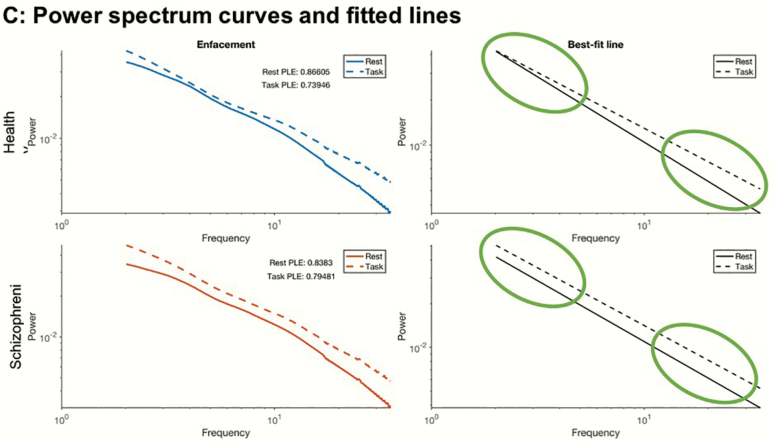
Next, given the known involvement of the resting state in self-referential task activity,32,37,41–43 we calculated the relative difference between task (enfacement illusion and auditory oddball task) and rest in both ACW and PLE, ie, rest-task difference. We observed significantly lower task-rest differences in both ACW and PLE in specifically the Enfacement illusion (but not the auditory oddball task) among the SCZ (figures 2a and 2b; P = .0370 and .0020, respectively). Power spectra and best-fit line (figure 2c) did not show any change from rest to task in both ACW and PLE in SCZ, whereas there was a clear difference in HC (see circles on the right in figure 2c; see supplementary material for further results): during the task, HC tilted their slow-fast frequency fractal power balance toward faster frequencies, whereas SCH exhibited no such shift (figure 2c).
Fig. 2.
Altered (A) autocorrelation window (ACW) and (B) power-law exponent (PLE) in the difference between Enfacement illusion and resting state. Scalp topographies of the ACW and PLE values are shown on top, and violin plots of the average values are shown at the bottom. The 2 left topoplots (for the 2 groups) represent the task values minus the resting-state values. The rightmost topoplot represents the group differences in this task-rest difference. White dots in the rightmost topoplot represent electrodes, which form part of the significant cluster. Black dots in this plot represent electrodes with significant results at the channel level, but which did not form part of the cluster. Violin plots show the average value of ACW or PLE over only the white electrodes. (C) Fractal power spectra of healthy and schizophrenia participants in rest and task states (x-axis: frequency range; y-axis: power). The actual data of fractal power spectra (extracted from the IRASA method) are shown left, while the best-fit lines (as plotted onto the data) used to estimate the PLE are shown right. The slope of the curve, which defines the PLE value, changes from rest to task in the healthy subjects (upper part) but not in the schizophrenia participants (lower part)—circles (on the right) indicate the shift in the slope from rest to task in the slower (upper circle) and faster (lower circle) frequency ranges. The rest and task curves are mostly parallel in both slower and faster frequency ranges in schizophrenia participants (lower right). Healthy subjects, in contrast, show deviating curves with power increase in faster frequencies (relative to slower frequencies) during the task (relative to rest) (upper right).
Relation of Neuronal to Phenomenological and Psychopathological Levels—Moderation Analysis
ACW and PLE during Enfacement illusion were found to directly correlate with none of the 3 components in a direct way. To investigate indirect relationships, we applied a moderation model80 to test for the differential impact of high and low ACW/PLE values on the relationship of the self-disorder (first component) with negative symptoms (second component) and positive symptoms (third component). Hence, rather than targeting the direct relationship of ACW/PLE to each of our 3 psychopathological PCA components (EASE and PANSS), the moderation analysis tests whether different levels of ACW/PLE, ie, low and high, mediate the relationship between the 3 PCA components in different ways.
We observed a highly significantly different impact of low and high ACW/PLE values on the relationship of the first component (self-disorders) with the second component (negative symptoms). When ACW/PLE values are low, self-disorders correlate negatively with the negative symptom component (P = .02042 for ACW and P = .02752 for PLE). In contrast, the 2 psychopathological components correlate positively when the values of ACW/PLE are high (P = .0054 for ACW and P = .00068 for PLE) (see supplementary figure S2 and supplementary tables S3.1–S3.3 for the detailed results). These results were obtained only for the enfacement illusion task, whereas no significant moderation effects were observed when testing for the effects of ACW/PLE during the auditory oddball task on the 3 PCA components.
Discussion
We conducted an EEG rest and task study investigating the brain’s intrinsic neural timescale in order to relate temporal integration on the neuronal level to temporal integration of self on psychological and phenomenological levels. Main results are: (1) PCA revealed self-disorder as psychopathological component distinct from negative and positive symptoms; (2) longer ACW and higher PLE only in self-referential (Enfacement illusion) but not non-self-referential task (oddball); (3) reduced rest-task differences in ACW and PLE during the self-referential task; and (4) ACW and PLE values, ie, low or high, moderate the relation of basic self-disorder and negative symptoms.
We observed that ACW and PLE change only during the self-referential task but not during a non-self-referential task. Hence, temporal integration on the neuronal level in SCZ is primarily altered with respect to self-referential information on the psychological level. Given that self-referential information is closely associated with resting-state activity in healthy subjects,32,37,41,42 we tested for rest-task modulation of ACW and PLE. SCZ participants showed reduced rest-task difference: unlike healthy subjects, they could not modulate their resting-state activity during self-referential task by shifting their neural activity toward shorter time scales (shorter ACW) and faster frequency power (lower PLE).81,82
Our results highlight a key role for abnormal temporal integration in basic self-disorder of SCZ. Longer intrinsic neural timescales (longer ACW and higher PLE) may facilitate abnormally strong integration of stimuli across different time points which otherwise, in the healthy state, would be segregated. Abnormally long ACW (and high PLE) may mediate temporal integration of face pictures over longer duration during enfacement—more face picture are consecutively integrated with self rather than being temporally segregated from self and related to non-self. Our moderation analysis suggests that such abnormal temporal integration on neuronal and psychological levels is intimately related to the phenomenological level of basic self-disorder and its impact on negative symptoms.
Limitations
Our sample size is small but provides outstanding phenomenological, psychological, and neuronal evaluation that need to be complemented by more large-scale studies. EEG measurements were based on the recordings of the entire Enfacement illusion task rather than single trials, including facial recognition of both self and other (see56 and supplementary material for more details).
Conclusions
We show prolonged intrinsic neural timescale in EEG of SCZ during specifically self-referential processing including its relation to basic self-disorder and its modulation of negative symptoms. These findings support our background assumption that abnormal temporal integration could provide the link or “common currency” 21 of neuronal, psychological, and phenomenological alterations of self in SCZ. Our results suggest that psychopathological symptoms may fundamentally be based on dynamic, ie, temporal (and spatial) abnormalities (rather than being primarily cognitive deficits)—this reflects the recently introduced “Spatiotemporal Psychopathology.” 83–86
Supplementary Material
Acknowledgments
We are grateful to Soren Wainio-Theberge for his generous assistance in data analysis. Dr Northoff is grateful for funding provided by UMRF, uOBMRI, CIHR, and PSI as well as the Mental Health Centre Zhejiang University in Hangzhou/China. Dr Northoff has received funding from the Canada-UK Artificial Intelligence (AI) Initiative ‘The self as agent-environment nexus: crossing disciplinary boundaries to help human selves and anticipate artificial selves’ (ES/T01279X/1). Dr Northoff also received funding from the Canadian Institute of Health Research (No 4560). Dr Sandsten is grateful for funding received from the Region Zealand Psychiatry, Denmark.
References
- 1. Parnas J, Henriksen MG. Disordered self in the schizophrenia spectrum: a clinical and research perspective. Harv Rev Psychiatry. 2014;22(5):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parnas J. The core Gestalt of schizophrenia. World Psychiatry. 2012;11(2):67–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Koren D, Tzivoni Y, Schalit L, et al. . Basic self-disorders in adolescence predict schizophrenia spectrum disorders in young adulthood: a 7-year follow-up study among non-psychotic help-seeking adolescents. Schizophr Res. 2020;216:97–103. [DOI] [PubMed] [Google Scholar]
- 4. Parnas J, Møller P, Kircher T, et al. . EASE: Examination of Anomalous Self-Experience. Psychopathology 2005;38(5):236–258. [DOI] [PubMed] [Google Scholar]
- 5. Fuchs T. AS22-03 – the self in schizophrenia: Jaspers, Schneider and beyond. Eur Psychiatry. 2012;27:1.22153731 [Google Scholar]
- 6. Stanghellini G, Ballerini M, Presenza S, et al. . Psychopathology of lived time: abnormal time experience in persons with schizophrenia. Schizophr Bull. 2016;42(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Northoff G, Stanghellini G. How to link brain and experience? Spatiotemporal psychopathology of the lived body. Front Hum Neurosci. 2016;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs T. The temporal structure of intentionality and its disturbance in schizophrenia. Psychopathology 2007;40 (4):229–235. [DOI] [PubMed] [Google Scholar]
- 9. Giersch A, Poncelet PE, Capa RL, et al. . Disruption of information processing in schizophrenia: the time perspective. Schizophr Res Cogn. 2015;2(2):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giersch A, Lalanne L, Isope P. Implicit timing as the missing link between neurobiological and self disorders in schizophrenia? Front Hum Neurosci. 2016;10:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin B, Wittmann M, Franck N, Cermolacce M, Berna F, Giersch A. Temporal structure of consciousness and minimal self in schizophrenia. Front Psychol. 2014;5:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martin B, Franck N, Cermolacce M, et al. . Fragile temporal prediction in patients with schizophrenia is related to minimal self disorders. Sci Rep. 2017;7(1):8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin B, Franck N, Cermolacce M, Coull JT, Giersch A. Minimal self and timing disorders in schizophrenia: a case report. Front Hum Neurosci. 2018;12:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martin B, Franck N, Giersch A. A reflection upon methods to explore timing in patients with schizophrenia. Psych J. 2019;8(1):82–89. [DOI] [PubMed] [Google Scholar]
- 15. Mishara A, Giersch A. SU107. Disrupted continuity of subjective time in the milliseconds range in the self-disturbances of schizophrenia: convergence of experimental, phenomenological, and predictive coding accounts. Schizophr Bull. 2017;43:S199–S200. [Google Scholar]
- 16. Bleuler E. Dementia Praecox or the Group of Schizophrenias [in German]. Translated by Zinkin J. New York: International Universities; 1950 [1911]. [Google Scholar]
- 17. Jaspers K. Allgemeine Psychopathologie. 7th ed. Berlin, Germany: Springer Edn, University of Chicago Press; 1959/1963. [Google Scholar]
- 18. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29(3):427–444. [DOI] [PubMed] [Google Scholar]
- 19. Mudrik L, Faivre N, Koch C. Information integration without awareness. Trends Cogn Sci. 2014;18(9):488–496. [DOI] [PubMed] [Google Scholar]
- 20. Himberger KD, Chien HY, Honey CJ. Principles of temporal processing across the cortical hierarchy. Neuroscience 2018;389:161–174. [DOI] [PubMed] [Google Scholar]
- 21. Northoff G, Wainio-Theberge S, Evers K. Is temporo-spatial dynamics the “common currency” of brain and mind? In Quest of “Spatiotemporal Neuroscience” [published online ahead of print May 23, 2019]. Phys Life Rev. doi: 10.1016/j.plrev.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 22. James W. The Principles of Psychology. New York: Henry Holt & Company;1890. [Google Scholar]
- 23. Northoff G. The brain’s intrinsic activity and inner time consciousness in schizophrenia. World Psychiatry 2014;13(2):144–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Northoff G. Slow cortical potentials and “inner time consciousness” – a neuro-phenomenal hypothesis about the “width of present”. Int J Psychophysiol. 2016;103:174–184. [DOI] [PubMed] [Google Scholar]
- 25. Murray JD, Bernacchia A, Freedman DJ, et al. . A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci. 2014;17(12):1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gollo LL, Zalesky A, Hutchison RM, van den Heuvel M, Breakspear M. Dwelling quietly in the rich club: brain network determinants of slow cortical fluctuations. Philos Trans R Soc Lond. B Biol Sci. 2015;370(1668):20140165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gollo LL, Roberts JA, Cocchi L. Mapping how local perturbations influence systems-level brain dynamics. Neuroimage 2017;160:97–112. [DOI] [PubMed] [Google Scholar]
- 28. Chaudhuri R, Knoblauch K, Gariel MA, Kennedy H, Wang XJ. A large-scale circuit mechanism for hierarchical dynamical processing in the primate cortex. Neuron 2015;88(2):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Honey CJ, Thesen T, Donner TH, et al. . Slow cortical dynamics and the accumulation of information over long timescales. Neuron 2012;76(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cavanagh SE, Wallis JD, Kennerley SW, Hunt LT. Autocorrelation structure at rest predicts value correlates of single neurons during reward-guided choice. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bernacchia A, Seo H, Lee D, Wang XJ. A reservoir of time constants for memory traces in cortical neurons. Nat Neurosci. 2011;14(3):366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wolff A, Di Giovanni DA, Gómez-Pilar J, et al. . The temporal signature of self: temporal measures of resting-state EEG predict self-consciousness. Hum Brain Mapp. 2019;40(3):789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe T, Rees G, Masuda N. Atypical intrinsic neural timescale in autism. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Z, Liu X, Mashour GA, Hudetz AG. Timescales of intrinsic BOLD signal dynamics and functional connectivity in pharmacologic and neuropathologic states of unconsciousness. J Neurosci. 2018;38(9):2304–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. He BJ, Zempel JM, Snyder AZ, Raichle ME. The temporal structures and functional significance of scale-free brain activity. Neuron 2010;66(3):353–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He BJ. Scale-free brain activity: past, present, and future. Trends Cogn Sci. 2014;18(9):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang Z, Obara N, Davis HH 4th, Pokorny J, Northoff G. The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia 2016;82:161–170. [DOI] [PubMed] [Google Scholar]
- 38. Linkenkaer-Hansen K, Nikouline VV, Palva JM, Ilmoniemi RJ. Long-range temporal correlations and scaling behavior in human brain oscillations. J Neurosci. 2001;21(4):1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Palva S, Palva JM. Roles of brain criticality and multiscale oscillations in temporal predictions for sensorimotor processing. Trends Neurosci. 2018;41(10):729–743. [DOI] [PubMed] [Google Scholar]
- 40. Huang Z, Zhang J, Longtin A, et al. . Is there a nonadditive interaction between spontaneous and evoked activity? Phase-dependence and its relation to the temporal structure of scale-free brain activity. Cereb Cortex. 2017;27(2):1037–1059. [DOI] [PubMed] [Google Scholar]
- 41. Davey CG, Pujol J, Harrison BJ. Mapping the self in the brain’s default mode network. Neuroimage 2016;132:390–397. [DOI] [PubMed] [Google Scholar]
- 42. Qin P, Northoff G. How is our self related to midline regions and the default-mode network? Neuroimage 2011;57(3):1221–1233. [DOI] [PubMed] [Google Scholar]
- 43. Bai Y, Nakao T, Xu J, et al. . Resting state glutamate predicts elevated pre-stimulus alpha during self-relatedness: a combined EEG-MRS study on “rest-self overlap”. Soc Neurosci. 2016;11(3):249–263. [DOI] [PubMed] [Google Scholar]
- 44. Damme KSF, Pelletier-Baldelli A, Cowan HR, Orr JM, Mittal VA. Distinct and opposite profiles of connectivity during self-reference task and rest in youth at clinical high risk for psychosis. Hum Brain Mapp. 2019;40(11):3254–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34(6):935–946. [DOI] [PubMed] [Google Scholar]
- 46. Potvin S, Gamache L, Lungu O. A functional neuroimaging meta-analysis of self-related processing in schizophrenia. Front Neurol. 2019;10:990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Taylor SF, Welsh RC, Chen AC, Velander AJ, Liberzon I. Medial frontal hyperactivity in reality distortion. Biol Psychiatry. 2007;61(10):1171–1178. [DOI] [PubMed] [Google Scholar]
- 48. Martin EA, Siegle GJ, Steinhauer SR, Condray R. Timing matters in elaborative processing of positive stimuli: gamma band reactivity in schizophrenia compared to depression and healthy adults. Schizophr Res. 2019;204:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson B, Lavoie S, Gawęda Ł, et al. . The neurophenomenology of early psychosis: an integrative empirical study. Conscious Cogn. 2020;77:102845. [DOI] [PubMed] [Google Scholar]
- 50. Metzler S, Theodoridou A, Aleksandrowicz A, et al. . Evaluation of trait adjectives and ego pathology in schizophrenia: an N400 study. Psychiatry Res. 2014;215(3):533–539. [DOI] [PubMed] [Google Scholar]
- 51. Pinheiro AP, Rezaii N, Rauber A, Nestor PG, Spencer KM, Niznikiewicz M. Emotional self-other voice processing in schizophrenia and its relationship with hallucinations: ERP evidence. Psychophysiology 2017;54(9):1252–1265. [DOI] [PubMed] [Google Scholar]
- 52. Jia S, Liu M, Huang P, et al. . Abnormal alpha rhythm during self-referential processing in schizophrenia patients. Front Psychiatry. 2019;10:691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murphy M, Stickgold R, Öngür D. Electroencephalogram microstate abnormalities in early-course psychosis. Biol Psychiatry. 2020;5(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tsakiris M. Looking for myself: current multisensory input alters self-face recognition. PLoS One. 2008;3(12):e4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tajadura-Jiménez A, Longo MR, Coleman R, Tsakiris M. The person in the mirror: using the enfacement illusion to investigate the experiential structure of self-identification. Conscious Cogn. 2012;21(4):1725–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sandsten KE, Nordgaard J, Kjaer TW, et al. . Altered self-recognition in patients with schizophrenia. Schizophr Res. 2020;218:116–123. [DOI] [PubMed] [Google Scholar]
- 57. Ferroni F, Ardizzi M, Sestito M, et al. . Shared multisensory experience affects Others’ boundary: the enfacement illusion in schizophrenia. Schizophr Res. 2019;206:225–235. [DOI] [PubMed] [Google Scholar]
- 58. Spencer KM, Ghorashi S. Oscillatory dynamics of Gestalt perception in schizophrenia revisited. Front Psychol. 2014;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hirano Y, Oribe N, Kanba S, Onitsuka T, Nestor PG, Spencer KM. Spontaneous gamma activity in schizophrenia. JAMA Psychiatry. 2015;72(8):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hirano Y, Oribe N, Onitsuka T, et al. . T13. Progressive spontaneous and synchrony gamma-band oscillation deficits in first episode schizophrenia. Schizophr Bull 2018;44:S117–S118. [Google Scholar]
- 61. Goldstein MR, Peterson MJ, Sanguinetti JL, Tononi G, Ferrarelli F. Topographic deficits in alpha-range resting EEG activity and steady state visual evoked responses in schizophrenia. Schizophr Res. 2015;168(1-2):145–152. [DOI] [PubMed] [Google Scholar]
- 62. Jang KI, Oh J, Jung W, et al. . Unsuccessful reduction of high-frequency alpha activity during cognitive activation in schizophrenia. Psychiatry Clin Neurosci. 2019;73(3):132–139. [DOI] [PubMed] [Google Scholar]
- 63. Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11(2):100–113. [DOI] [PubMed] [Google Scholar]
- 64. Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry. 2015;77(12):1001–1009. [DOI] [PubMed] [Google Scholar]
- 65. Gomez-Pilar J, de Luis-García R, Lubeiro A, et al. . Deficits of entropy modulation in schizophrenia are predicted by functional connectivity strength in the theta band and structural clustering. Neuroimage Clin. 2018;18:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Damiani S, Scalabrini A, Gomez-Pilar J, Brondino N, Northoff G. Increased scale-free dynamics in salience network in adult high-functioning autism. Neuroimage Clin. 2019;21:101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends Neurosci. 2010;33(6):277–284. [DOI] [PubMed] [Google Scholar]
- 68. Magno E, Yeap S, Thakore JH, Garavan H, De Sanctis P, Foxe JJ. Are auditory-evoked frequency and duration mismatch negativity deficits endophenotypic for schizophrenia? High-density electrical mapping in clinically unaffected first-degree relatives and first-episode and chronic schizophrenia. Biol Psychiatry. 2008;64(5):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brockhaus-Dumke A, Tendolkar I, Pukrop R, Schultze-Lutter F, Klosterkötter J, Ruhrmann S. Impaired mismatch negativity generation in prodromal subjects and patients with schizophrenia. Schizophr Res. 2005;73(2-3):297–310. [DOI] [PubMed] [Google Scholar]
- 70. Michie PT. What has MMN revealed about the auditory system in schizophrenia? Int J Psychophysiol. 2001;42 (2):177–194. [DOI] [PubMed] [Google Scholar]
- 71. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 72. McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–770. [DOI] [PubMed] [Google Scholar]
- 73. Gross G, Huber G, Klosterkotter J, Linz M.. Bonner Skala für die Beurteilung von Basissymptomen. Berlin: Springer;1987. [Google Scholar]
- 74. Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, Levin AR. The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): standardized processing software for developmental and high-artifact data. Front Neurosci. 2018;12:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- 76. Winkler I, Haufe S, Tangermann M. Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav Brain Funct. 2011;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nolan H, Whelan R, Reilly RB. FASTER: fully automated statistical thresholding for EEG artifact rejection. J Neurosci Methods. 2010;192(1):152–162. [DOI] [PubMed] [Google Scholar]
- 78. Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 79. Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ferri F, Nikolova YS, Perrucci MG, et al. . A neural “Tuning Curve” for multisensory experience and cognitive-perceptual schizotypy. Schizophr Bull. 2017;43(4):801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog Neurobiol. 2016;145-146:26–45. [DOI] [PubMed] [Google Scholar]
- 82. Northoff G. Is the self a higher-order or fundamental function of the brain? The “basis model of self-specificity” and its encoding by the brain’s spontaneous activity. Cogn Neurosci. 2016;7(1-4):203–222. [DOI] [PubMed] [Google Scholar]
- 83. Northoff G. Spatiotemporal psychopathology I: no rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. J Affect Disord. 2016;190:854–866. [DOI] [PubMed] [Google Scholar]
- 84. Northoff G. Spatiotemporal psychopathology II: how does a psychopathology of the brain’s resting state look like? Spatiotemporal approach and the history of psychopathology. J Affect Disord. 2016;190:867–879. [DOI] [PubMed] [Google Scholar]
- 85. Northoff G. The brain’s spontaneous activity and its psychopathological symptoms–“Spatiotemporal binding and integration”. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt B):81–90. [DOI] [PubMed] [Google Scholar]
- 86. Fingelkurts AA, Fingelkurts AA. Brain space and time in mental disorders: paradigm shift in biological psychiatry. Int J Psychiatry Med. 2019;54(1):53–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.