Abstract
Background
Functional connectivity abnormalities between Broca’s and Wernicke’s areas and the putamen revealed by functional magnetic resonance imaging (fMRI) are related to auditory hallucinations (AH). In long-term schizophrenia, reduced white matter structural integrity revealed by diffusion imaging in left arcuate fasciculus (connecting Broca’s and Wernicke’s areas) is likely related to AH. The structural integrity of connections with putamen and their relation to AH are unknown. Little is known about this relationship in first-episode psychosis (FEP), although auditory transcallosal connections were reported to play a role. White matter in the Broca’s-Wernicke’s-putamen language-related circuit and auditory transcallosal fibers was examined to investigate associations with AH in FEP.
Methods
White matter connectivity was measured in 40 FEP and 32 matched HC using generalized fractional anisotropy (gFA) derived from diffusion spectrum imaging (DSI).
Results
FEP and HC did not differ in gFA in any fiber bundle. In FEP, AH severity was significantly inversely related to gFA in auditory transcallosal fibers and left arcuate fasciculus. Although the right hemisphere arcuate fasciculus-AH association did not attain significance, the left and right arcuate fasciculus associations were not significantly different.
Conclusions
Despite overall normal gFA in FEP, AH severity was significantly related to gFA in transcallosal auditory fibers and the left hemisphere connection between Broca’s and Wernicke’s areas. Other bilateral tracts’ gFA were weakly associated with AH. At the first psychotic episode, AH are more robustly associated with left hemisphere arcuate fasciculus and interhemispheric auditory fibers microstructural deficits, likely reflecting mistiming of information flow between language-related cortical centers.
Keywords: first-episode psychosis, auditory hallucination, arcuate fasciculus, Broca’s area, Wernicke’s area, putamen, transcallosal fibers
Introduction
Inferior frontal gyrus (IFG) and posterior temporal/parietal areas are critical for language-related functioning.1–5 IFG is crucial for verbal fluency,6 and is thought to control guided semantic access.7 Increased functional magnetic resonance imaging (fMRI) activity in Broca’s area in IFG is associated with decreased activity in Wernicke’s area in posterior temporal/parietal cortex,8 indicating inhibitory control from IFG to semantic memory access via posterior temporal/parietal cortex.
Basal activity and functional connectivity between these areas is impaired in schizophrenia (Sz). Using fMRI, Kuperberg et al9 showed a failure of Broca’s-to-Wernicke’s inhibition in Sz on a semantic association task, with widespread temporal cortex over-activity. Crossley et al10 indicated a similar failure of temporal area deactivation on a working memory task in first-episode schizophrenia (FESz) and clinical high risk for psychosis individuals (CHR). Basal over-activity in the left hemisphere dominant language/semantic network has also been observed in CHR subjects, and over-activation of left superior temporal gyrus and left IFG was greater in those that transitioned to psychosis.11
Frontal-temporal functional dysconnectivity is relevant for auditory hallucinations (AH). AH are associated with increased basal activity in left Wernicke’s area, primary auditory cortex,12–16 and Broca’s area.13 Increased fMRI functional connectivity between these areas has been demonstrated in AH.17 A lack of information flow during internal speech from left Wernicke’s and right hemisphere homologues of Broca’s and Wernicke’s areas to left Broca’s area may characterize AH.18 Increased basal blood flow in left hemisphere auditory cortices normalized with resolution of AH but returned with relapse.19 Further, brain stimulation with repetitive Transcortical Magnetic Stimulation (rTMS) produces reductions of AH and basal activity in left temporal/parietal language areas20–22 in most medication-resistant hallucinators. Finally, basal activity demonstrated by arterial spin labeling is reduced in Wernicke’s area, Broca’s area, and primary auditory cortex after rTMS for AH.16 Thus, AH are associated with functional connectivity abnormalities in the language/semantic cortical circuit that may lead to over-activation within left Wernicke’s area due to failure of Broca’s area controlled inhibition.
The arcuate fasciculus, the major fiber bundle connecting Broca’s and Wernicke’s areas, may underlie such functional dysconnectivity. What arcuate fasciculus structural abnormalities impact the functional connectivity between Broca’s and Wernicke’s areas remain unknown. Strik and colleagues14 proposed that Broca’s and Wernicke’s areas were hyper-connected, demonstrating increased functional anisotropy (FA) using Diffusion Tensor Imaging (DTI) in hallucinators (AH+) vs non-hallucinators (AH-).23 On the other hand, Ford and colleagues speculated a failure of corollary discharge from frontal cortex to dampen auditory cortex activity leads to AH.24–26 Whitford et al27 showed associations between reduced arcuate fasciculus integrity and a failure of corollary discharge in early course Sz. Thus, both structural hyper- and hypo-connectivity of arcuate fasciculus have been proposed as the underlying white matter abnormality causing AH. Most studies of arcuate fasciculus integrity in Sz independent of the presence of AH have found decreased FA in the left arcuate fasciculus (table 1). Most studies find lower FA in the left arcuate fasciculus in AH+ compared to AH-, although some report arcuate fasciculus hyper-connectivity in AH+ (table 1).
Table 1.
Arcuate Fasciculus Findings in Sz
| Reference | Participants | Field Strength | Findings |
|---|---|---|---|
| Abdul-Rahman et al28 | 32 Sz 44 HC |
3T | Sz had lower FA in left frontal arcuate fasciculus Sz had higher FA and AD in left temporal arcuate fasciculus FA and AD correlated positively with severe positive symptoms |
| Boos et al29 | 126 Sz 123 nonpsychotic siblings 109 HC |
1.5T | FA in arcuate fasciculus negatively correlated with PANNS positive, negative, general, and total symptom scores Siblings had higher FA than Sz and HC |
| Burns et al30 | 30 Sz 30 HC |
1.5T | Sz had lower FA in left arcuate fasciculus |
| Kubicki et al31 | 21 Sz 26 HC |
1.5T | Sz had lower FA in left arcuate fasciculus |
| Catani et al32 | 17 AH+ 11 AH- 59 HC |
1.5T | Sz had lower FA in left and right arcuate fasciculus AH+ had lower FA in left and right arcuate fasciculus AH- had lower FA in left and right arcuate fasciculus |
| Ćurčić-Blake18 | 17 AH+ 14 AH- 14 HC |
3T | AH+ had lower FA in anterior left and right arcuate fasciculus |
| de Weijer et al33 | 44 AH+ 42 HC |
3T | AH+ had lower FA in left and right arcuate fasciculus |
| de Weijer et al34 | 35 Sz AH+ 35 nonpsychotic AH+ 36 HC |
3T | Sz AH+ had lower FA in left arcuate fasciculus than HC and nonpsychotic AH+ |
| Hubl et al14 | 13 AH+ 13 AH- 13 HC |
1.5T | FA higher in right vs left arcuate fasciculus AH+ had higher FA in left lateral arcuate fasciculus than AH- or HC AH+ and AH- had lower FA in medial left arcuate fasciculus |
| McCarthy-Jones et al35 | 18 Current AH+ 21 Remitted AH+ 40 non-auditory hallucinations 34 no hallucinations 40 HC |
1.5T | AH+ had lower FA in left arcuate fasciculus versus HC or AH- AH+ had lower FA in left arcuate fasciculus than AH- w/ hallucinations in other modalities FA of left arcuate fasciculus had nonlinear relationship with AH state |
| Phillips36 | 23 Sz 22 HC |
1.5T | Sz had lower FA in left arcuate fasciculus Sz had lower volume of left arcuate fasciculus |
| Rotarska-Jagiela et al37 | 24 AH+ paranoid Sz 24 HC |
3T | Sz had higher FA in arcuate fasciculus than HC FA in left arcuate fasciculus correlated positively with positive PANSS factor and AH severity |
| Seok et al38 | 15 AH+ 15 AH- 22 HC |
1.5T | Both AH+ and AH- had lower FA in the anterior superior longitudinal fasciculus |
| Shergill et al39 | 9 Current AH+ 17 Remitted AH+ 7 AH- 40 HC |
1.5T | Sz had lower FA in right frontal and temporal-parietal portions of superior longitudinal fasciculus FA of lateral aspect of left and right superior longitudinal fasciculus positively correlated with “propensity for auditory hallucinations” |
Note: Sz, schizophrenia; FESz, first-episode schizophrenia; HC, healthy comparison subjects; AH+, hallucinations present; AH-, no hallucinations; T, Tesla; FA, fractional anisotropy; AD, axial diffusivity; PANSS, Positive and negative Syndrome Scale.
FESz show more subtle white matter abnormalities (ref. 40, table 2). Most studies examining the arcuate fasciculus observed no abnormalities in FESz,41,42 although lower diffusivity in the left arcuate fasciculus, the left posterior superior temporal gyrus, and superior longitudinal fasciculus have been reported.43–45 No studies compared FESz hallucinators and non-hallucinators.
Table 2.
Arcuate Fasciculus Findings in Early Schizophrenia
| Reference | Participants | Field Strength | Findings |
|---|---|---|---|
| Cheung et al41 | 25 FESz 26 HC |
1.5T | No FA differences |
| Cheung et al43 | 34 FESz 32 HC Includes participants from Cheung et al43 |
1.5T | FESz had lower FA in left superior temporal gyrus (right frontal lobe, left anterior cingulate gyrus, etc.) FESz with more positive symptoms had FA values closer to controls |
| Douaud et al44 | 25 adolescent onset Sz 25 HC |
1.5T | Sz had reduced FA in left arcuate fasciculus |
| Karlsgodt et al46 | 12 recent-onset Sz 17 HC |
1.5T | Sz had lower FA in superior longitudinal fasciculus; particular deficit in left hemisphere |
| Mandl et al47 | 16 FESz 23 HC |
1.5T | Group differences between FESz and HC in left arcuate fasciculus from combined FA, mean diffusivity, magnetization transfer ratio values |
| Peters et al42 | 10 FESz (first or second episode) 10 UHR 10 HC |
3T | No FA differences |
| Szeszko et al45 | 10 FESz 13 HC |
1.5T | FESz had lower FA in left posterior superior temporal gyrus (primary auditory cortex) |
| Whitford et al27 | 51 Early Course Sz 40 CHR 59 HC |
3T | ESz had lower FA in arcuate fasciculus than CHR and HC ESz had higher radial diffusivity in arcuate fasciculus than CHR and HC |
Note: Sz, schizophrenia; ESz, early course schizophrenia; FESz, first-episode schizophrenia; HC, healthy comparison subjects; AH+, hallucinations present; AH-, no hallucinations; UHR, ultra-high risk for psychosis; CHR, clinical high risk for psychosis.
Because Sz shows progressive white matter impairment throughout life,48 individuals with long-term Sz likely have greater structural deterioration compared to FESz. The rate of white matter decline with age is 60% steeper in Sz than in neurotypical aging,49 resulting in greater differences in FA as the disease progresses. Examination of specific neurobiological circuits that relate to symptoms present at disease onset will help determine whether white matter deficits are proximal to symptom emergence or develop as a disease consequence. We hypothesize AH severity in early disease course will correlate with structural integrity in the left arcuate fasciculus.
However, while the arcuate fasciculus is a critical tract connecting 2 central hubs of the left hemisphere dominant language system, other areas are involved in AH and may show physical dysconnectivity. Hoffman et al17 proposed that fMRI-demonstrated functional dysconnectivity between 3 legs of a Broca’s-Wernicke’s-putamen circuit led to AH. Although the putamen is traditionally thought of as a motor area,50,51 it has a role in non-motoric aspects of speech such as syntax,52 and reductions of left putamen volume occur in progressive semantic dementia.53 Vinas-Gausch & Wu50 argued that putamen influenced language production via connections with Broca’s area and motor cortices, but also showed extensive connections to areas involved in syntactic, phonological, and semantic processing, including left Broca’s area, left Wernicke’s area, left inferior parietal lobule, and left middle temporal gyrus. Thus, putamen plays a role in more cognitive aspects of language, although its precise role is unknown. Later single voxel connectivity analysis from the Hoffman group suggests AH are related to abnormal activity in the left language circuit and the default mode network, with hubs in Broca’s, Wernicke’s, and left putamen.54 Using fMRI, Cui et al55 found reduced left putamen low-frequency activity in AH+ vs AH- FESz. Relatedly, Dandash et al56 reported (among other findings) increased resting-state functional connectivity between putamen and left superior temporal gyrus in CHR. Thus, there is evidence for pathophysiology in Broca’s-Wernicke’s-putamen in AH+ Sz, even early in disease course.
Few studies have looked at physical connections between Broca’s area and putamen or Wernicke’s area and putamen. Quan et al57 reported reduced FA in the left hemisphere tracts connecting the striatum (putamen, caudate, and nucleus accumbens) and the IFG (pars opercularis, pars tirangularis, pars orbitalis). Bloemen et al58 reported reduced white matter integrity in left superior temporal areas and adjacent to right putamen (among other areas) in ultra-high risk individuals that later transitioned to psychosis. Still, putamen structural connectivity in Sz and its role in AH is little studied.
Finally, interhemispheric auditory cortex communication is likely also affected in AH, although results have been equivocal. Auditory transcallosal fibers showed increased FA in AH+ FESz,59 but others report reduced transcallosal FA in AH+.37,60,61 Definitive knowledge is lacking.
In summary, 2 major circuits, the Broca’s area-Wernicke’s area-putamen circuit and the interhemispheric auditory connections, have emerged as likely culprits in the genesis of AH. Although somewhat equivocal, the weight of evidence for arcuate fasciculus and auditory transcallosal fibers suggests reduced structural integrity. As mentioned above, left Wernicke’s area over-activation may be a final common pathway for AH. Paradoxically, reduced physical connectivity may lead to functional over-activity from inhibitory decoupling, and recent work has demonstrated increased fMRI functional connectivity following mild traumatic brain injury.62 In terms of structural integrity of white matter, relatively little is known about the connections between the hub areas of the “Hoffman Hallucination Circuit,” or in the connections between the right and left hemisphere auditory cortices with respect to hallucinations. To determine the integrity of these white matter pathways, we examined white matter integrity and AH status in first-episode psychosis (FEP) individuals, with the hypothesis of reduced FA in one or more of the pathways that correlated with AH severity.
Methods and Materials
Participants
Forty FEP individuals recruited from consecutive admissions to Western Psychiatric Hospital inpatient and outpatient services were compared with 32 matched HC. FEP individuals were within 2 months of their first clinical contact for psychosis, and met criteria for a full psychotic episode. Twenty-one FEP individuals were diagnosed with Sz (paranoid = 11, undifferentiated =10), 7 with psychotic disorder NOS, 3 with schizoaffective disorder (depressed subtype = 2, bipolar subtype = 1), 2 with schizophreniform disorder, 3 with major depressive disorder with psychotic features, and 4 with Bipolar I with psychotic features. FEP had less than 2 months of lifetime antipsychotic medication exposure. Twelve FEP (30.0%) were unmedicated.
None of the participants had a history of concussion or head injury with sequelae, history of alcohol or drug dependence, detox in the last 5 years, or neurological comorbidity. The 4-factor Hollingshead Scale was used to measure socioeconomic status (SES) in participants and in their parents. Groups were matched for age, gender, handedness, premorbid estimates of intelligence based on the Wechsler Abbreviated Scale of Intelligence (WASI), and parental SES (table 3). Participants provided informed consent and were paid for participation. Procedures were approved by the University of Pittsburgh IRB.
Table 3.
Participant Demographic, Clinical, and Neuropsychological Measures
| FEP | HC | Test Statistic | P | AH+ | AH- | Test Statistic | P | |
|---|---|---|---|---|---|---|---|---|
| N | 40 | 32 | - | - | 23 | 17 | - | - |
| Age | 22.41 ± 4.49 | 21.00 ± 3.57 | t 70 = 1.43 | .153 | 21.75 ± 458 | 23.29 ± 4.33 | t 38 = 1.07 | .288 |
| Gender | 28 M / 12 F | 22 M / 10 F | χ 2 = .013 | .909 | 18 M / 5 F | 10 M / 7 F | χ 2 = 0.1759 | .185 |
| Handedness | 33 R / 5 L / 1 M / 1 Missing | 29 R / 3 L | χ 2 = .189 | .595 | 18 R / 3 L / 1M / 1 Missing | 15 R / 2 L | χ 2 = 1.609 | .657 |
| SES | 32.03 ± 15.07 | 32.38 ± 12.97 | t 68 = −0.103 | .918 | 29.95 ± 14.12 | 34.59 ± 16.22 | t 36 = 0.942 | .353 |
| Parental SES | 43.45 ± 15.15 | 48.44 ± 13.36 | t 67 = −1.44 | .154 | 43.48 ± 12.31 | 43.41 ± 18.68 | t (24.54) = −0.013 | .990 |
| Education (y) | 13.21 ± 2.62 | 13.69 ± 2.79 | t 69 = −0.750 | .456 | 12.95 ± 2.92 | 13.53 ± 2.21 | t 37 = 0.675 | .504 |
| WASI IQ | 105.45 ± 14.85 | 107.34 ± 9.46 | t (66.92) = −0.626 | .533 | 107.13 ± 15.21 | 103.18 ± 14.49 | t 38 = −0.829 | .412 |
| MCCB total | 39.25 ± 15.76 | 48.59 ± 7.26 | t (50.46) = −3.20 | .002 | 39.32 ± 16.18 | 39.14 ± 15.67 | t 34 = −0.032 | .975 |
| Processing speed | 44.49 ± 15.86 | 50.88 ± 7.37 | t (52.43) = −2.191 | .033 | 44.68 ± 17.93 | 44.20 ± 12.84 | t 35 = −0.089 | .929 |
| Attention/vigilance | 41.22 ± 12.24 | 46.47 ± 8.68 | t (63.01) = −2.055 | .044 | 40.68 ± 12.86 | 42.07 ± 11.63 | t 34 = 0.328 | .745 |
| Working memory | 42.68 ± 13.29 | 45.56 ± 9.42 | t (64.62) = −1.051 | .297 | 42.55 ± 14.35 | 42.87 ± 12.06 | t 35 = 0.071 | .944 |
| Verbal learning | 45.14 ± 11.67 | 50.94 ± 9.44 | t 67 = −2.247 | .028 | 44.86 ± 11.66 | 45.53 ± 12.09 | t 35 = 0.169 | .847 |
| Visual learning | 40.87 ± 13.44 | 45.59 ± 7.06 | t (56.00) = −1.87 | .066 | 39.64 ± 13.13 | 42.60 ± 14.16 | t 35 = 0.653 | .518 |
| Reasoning | 46.57 ± 11.32 | 50.91 ± 6.35 | t (58.04) = −2.00 | .050 | 48.00 ± 11.23 | 44.47 ± 11.49 | t 35 = −0.931 | .358 |
| Social cognition | 43.70 ± 14.39 | 54.50 ± 7.86 | t (57.34) = −3.94 | .000 | 44.00 ± 15.76 | 43.27 ± 12.62 | t 35 = −0.150 | .881 |
| PANSS total | 71.54 ± 15.05 | - | - | - | 76.50 ± 15.95 | 64.93 ± 11.09 | t 33 = −2.403 | .022 |
| PANSS positive | 19.06 ± 5.63 | - | - | - | 20.85 ± 5.44 | 16.67 ± 5.09 | t 33 = −2.31 | .027 |
| PANSS negative | 15.54 ± 5.29 | - | - | - | 17.15 ± 5.50 | 13.40 ± 4.27 | t 33 = −0.2189 | .036 |
| SANS global | 9.25 ± 2.96 | - | - | - | 10.00 ± 3.06 | 8.24 ± 2.54 | t 38 = −1.91 | .064 |
| SAPS global | 6.18 ± 3.35 | - | - | - | 7.26 ± 3.09 | 4.71 ± 3.12 | t 38 = −2.55 | .015 |
| GF:S current | 5.83 ± 1.85 | 9.02 ± 0.204 | t (40.21) = −10.81 | .000 | 5.39 ± 1.83 | 6.41 ± 1.77 | t 38 = 1.77 | .085 |
| Low | 5.78 ± 1.87 | 8.98 ± 0.157 | t (39.71) = −10.78 | .000 | 5.39 ± 1.97 | 6.29 ± 1.65 | t 38 = 1.53 | .134 |
| High | 7.48 ± 1.20 | 9.03 ± 0.180 | t (41.25) = −8.01 | .000 | 7.17 ± 1.19 | 7.82 ± 1.11 | t 38 = 1.91 | .064 |
| GF:R current | 5.95 ± 2.28 | 9.00 ± 0.408 | t (42.41) = −8.31 | .000 | 5.65 ± 2.50 | 6.35 ± 1.93 | t 38 = 0.962 | .342 |
| Low | 5.78 ± 2.20 | 8.95 ± 0.373 | t (41.87) = −8.96 | .000 | 5.52 ± 2.45 | 6.12 ± 1.83 | t 38 = 0.140 | .404 |
| High | 7.55 ± 1.36 | 9.07 ± 0.170 | t (40.58) = −6.98 | .000 | 7.34 ± 1.42 | 7.82 ± 1.23 | t 38 = 0.129 | .279 |
| SFS | 99.24 ± 11.31 | 104.94 ± 12.56 | t 67 = −1.98 | .055 | 97.07 ± 11.86 | 102.57 ± 9.86 | t 36 = 1.49 | .145 |
Note: FEP, first-episode psychosis; HC, healthy comparison subjects; AH+, FEP with hallucinations; AH-, FEP without hallucinations; SES, Socioeconomic status; WASI IQ, Wechsler Abbreviated Scale of Intelligence Intelligence Quotient; MCCB, Matrics Consensus Cognitive battery; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; GF:S = Global functioning: Social; GF:R, Global functioning: Role; SFS, Social Functioning Scale.
Diagnostic Assessments
Diagnosis was based on the Structured Clinical Interview for DSM-IV (SCID-P). Symptoms were rated using the Positive and Negative Symptom Scale (PANSS), Scale for Assessment of Positive Symptoms (SAPS), and Scale for Assessment of Negative Symptoms (SANS, table 3). AH severity was based on the PANSS hallucination score. Questionable hallucinators (ie, participants appearing to be responding to internal stimuli) were grouped with AH+.
Neuropsychological Tests
Participants completed the MATRICS Cognitive Consensus Battery (MCCB) and the WASI. Social functioning was assessed with the Global Assessment Scale (GAS), Global Functioning: Social and Role scales (GF:S, GF:R), and the Social Functioning Scales (SFS, table 3).
Image Acquisition
T1-weighted structural images were obtained on a 3T Siemens TIM Trio MRI system using a MPRAGE sequence. Contiguous slices were acquired in the sagittal plane with 1 mm3 voxel resolution (TR/TE/TI [ms] = 2530/1.74, 3.6, 5.46, 7.32/1260, 7° flip angle, 256 × 256 × 176 acquisition matrixes, FOV = 256 × 256 mm, GRAPPA acceleration factor = 2, acquisition time 6:02).
Diffusion spectrum imaging (DSI) was acquired in the same magnet and session using 101 diffusion-encoding directions (bmax = 4000 s/mm2, 1.8 mm in-plane resolution, 1.8 mm slice thickness, TR/TE = 9100/142 ms, 12 b = 0 s/mm2 images, multiband acceleration factor = 3, 113 images total, acquisition time 8:32). Images were reconstructed with generalized q-Sampling imaging with a sampling length ratio of 1.2, half-sphere DSI-scheme, and restricted diffusion imaging.60
DSI Analysis
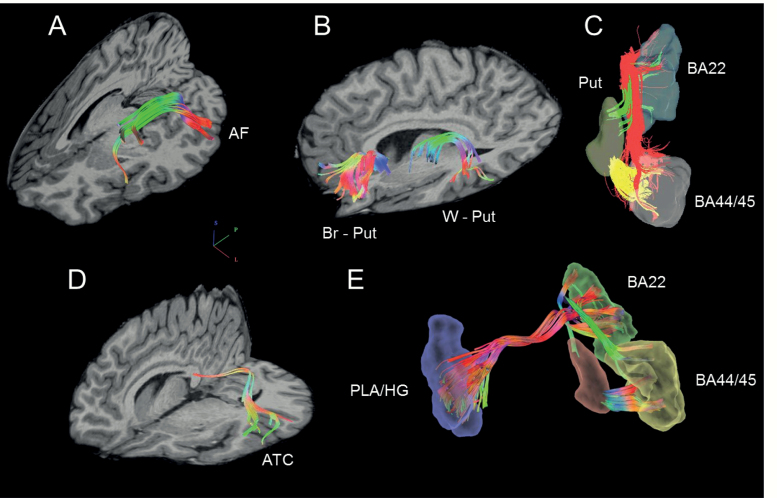
Diffusion fiber tracking utilized DSI_Studio software using a deterministic fiber tracking algorithm,63 with a step size of 1.0 mm, minimum fiber length of 20 mm, and turning angle threshold of 75. Progressive direction estimates were weighted 80% by neighboring fibers and 20% by the previous step. Fibers were thresholded at a quantitative anisotropy value of .015-.040. The 3 fiber tracts in each hemisphere that connect Broca’s area to Wernicke’s area (arcuate fasciculus), Broca’s area to putamen, and Wernicke’s area to putamen were isolated, as were the transcallosal fibers connecting auditory cortex (figure 1). Tracts were identified by seeding a predetermined number of fibers previously shown to provide full coverage of each tract. The arcuate fasciculus was defined as 5000 fibers between Broca’s (Brodmann’s Areas 44/45) and Wernicke’s (Brodmann’s Area 22). Fibers were seeded in either Broca’s or Wernicke’s and had to terminate in either area. The tract from Broca’s to putamen was defined as 5000 fibers between Broca’s and the putamen (Harvard Oxford Subcortical Atlas). Fibers were seeded in either Broca’s or the putamen and had to terminate in either area. The tract from Wernicke’s to putamen was defined as 5000 fibers between Wernicke’s and the putamen. Fibers were seeded in either Wernicke’s or the putamen and had to terminate in either area. The auditory transcallosal fibers were defined as 1000 fibers passing through the posterior third of the corpus callosum and ending bilaterally in Brodmann’s area 22, Heschl’s gyrus, or planum temporale. Manual editing was performed for quality control. When fibers were not part of the tract of interest (eg, inferior longitudinal fasciculus fibers were sometimes traced along with the arcuate fasciculus), they were manually removed.
Fig. 1.
The Tracts-of-Interest. (A) Arcuate Fasciculus (AF) connecting Broca’s area (Brodmann’s areas 44/45) and Wernicke’s area (Brodmann’s area 22) Colors indicate direction. (B) Broca’s area to putamen (Br-Put) and Wernicke’s area to putamen (W-Put). Colors indicate direction. (C) Hoffman hallucination circuit between Broca’s area, Wernicke’s area, and putamen (shown only for left hemisphere). Colors indicate different tracts. (D) Auditory transcallosal fibers (ATC) connecting Heschl’s gyrus (HG), planum temporale (PT), and Wernicke’s area (Brodmann’s area 22) bilaterally. Colors indicate direction. (E) Final distributed circuit (right hemisphere Broca’s area-Wernicke’s area-putamen tracts not shown).
Generalized FA (gFA) was used among DSI metrics as it approximates the widely used FA from DTI. DTI calculates the flow of water in each voxel along several dimensions and calculates a tensor for each voxel, akin to an average vector of the rate of flow within the voxel. FA is defined as the ratio of the standard deviation of flow in 3 dimensions (the main axis of flow and the 2 directions orthogonal to it) to the average flow variance (root mean square) within the voxel. DSI measures the density of diffusion. The DSI orientation distribution function indicates the directions of movement within a voxel and the density of flow within each direction, providing “sub-voxel” resolution of multiple “directionalities.” gFA is calculated as the standard deviation of the orientation density function to the root mean square estimate of the overall variance. Both FA and gFA are unitless and range between 0 and 1.
Analyses
Group demographics and neuropsychological scores were compared using t-tests and chi-squared tests where appropriate. DSI analyses for transcallosal fibers utilized one-way ANOVA, and for bilateral tracts utilized repeated-measures ANOVA, with group as the between-subjects factor, and hemisphere as the within-subjects’ factor. Planned comparisons of AH+ and AH- FEP subgroups and HC utilized Tukey’s Honestly Significant Difference. Correlations used Spearman’s rho. Significance was attained at P <.05.
Results
Demographics, Neuropsychological Function, and Clinical Symptoms
FEP and HC did not significantly differ in age, gender distribution, handedness, premorbid IQ, SES, or parental SES (table 3). FEP scored lower than HC on the MCCB composite score, consistent with the deleterious effects of psychosis on cognition, in the context of good premorbid IQ. FEP were generally more impaired in social functioning. AH+ and AH- did not differ significantly on any demographic, neuropsychological, or social functioning measure, although AH+ generally had greater symptoms (table 3) (Covarying total PANSS scores and medication did not change the differences between AH+ and AH- groups. Further, total PANSS and Positive and Negative factor scores did not correlate significantly with gFA in any tract.).
Auditory Transcallosal gFA
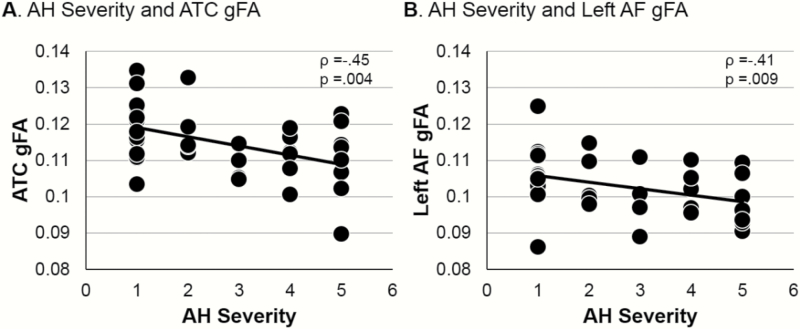
One-way ANOVA revealed that FEP and HC did not differ in gFA in auditory transcallosal fibers (F(1,70) = 0.34, P = .562, see table 4 for all gFA measures). However, among FEP, auditory transcallosal gFA correlated with hallucination severity (ρ = −0.45, P = .004, figure 2A, see table 5 for all correlations).
Table 4.
gFA of the Hoffman Hallucination Circuit Hub Connections
| FEP | HC | AH+ | AH- | |
|---|---|---|---|---|
| N | 40 | 32 | 23 | 17 |
| Auditory transcallosal | 0.115 ± 0.009 | 0.114 ± 0.008 | 0.112 ± 0.009 | 0.119 ± 0.008a |
| Left arcuate fasciculus | 0.103 ± 0.008 | 0.103 ± 0.009 | 0.101 ± 0.007 | 0.106 ± 0.008 |
| Right arcuate fasciculus | 0.103 ± 0.008 | 0.103 ± 0.006 | 0.102 ± 0.008 | 0.105 ± 0.007 |
| Left Broca’s area to putamen | 0.094 ± 0.006 | 0.097 ± 0.007 | 0.092 ± 0.004b | 0.096 ± 0.008 |
| Right Broca’s area to putamen | 0.093 ± 0.007 | 0.095 ± 0.008 | 0.093 ± 0.006 | 0.094 ± 0.008 |
| Left Wernicke’s area to putamen | 0.111 ± 0.008 | 0.111 ± 0.009 | 0.109 ± 0.009 | 0.113 ± 0.007 |
| Right Wernicke’s area to putamen | 0.105 ± 0.009 | 0.109 ± 0.010 | 0.103 ± 0.007b | 0.109 ± 0.009 |
Note: AH+, FEP with hallucinations; AH-, FEP without hallucinations; gFA, generalized fractional anisotropy; FEP, first-episode psychosis; HC, healthy comparison subjects.
aAH+ were significantly smaller than AH- via Tukey’s Honestly Significant Difference (HSD).
bAH+ were smaller than HC via Tukey’s HSD.
Fig. 2.
Scatterplots of auditory hallucination (AH) severity and gFA in FEP. (A) AH severity is related to auditory transcallosal gFA. (B) AH severity is related to left arcuate fasciculus (AF) gFA.
Table 5.
Pearson’s ρ Measures of Association Between Hallucinations and Each Tract gFA
| Auditory Transcallosal | Arcuate Fasciculus | Broca’s Area to Putamen | Wernicke’s Area to Putamen | |
|---|---|---|---|---|
|
−0.45
P = .004 |
||||
| Left Hemisphere |
−0.41
P = .009 |
−0.28 P = .084 |
−0.21 P = .196 |
|
| Right Hemisphere | −0.24 P = .141 |
−0.21 P = .194 |
−0.23 P = .157 |
Note: Subject sample size = 40. Nonparametric Pearson’s ρ is reported. P values are 2-tailed. Significant correlations are bolded. gFA, generalized fractional anisotropy. Auditory transcallosal fibers span both hemispheres.
Omnibus Analysis of gFA for Bilateral Tracts
Repeated-measures ANOVA of all bilateral tracts indicated differences in gFA among tracts (F(2,140) =119.30, P < .001, ε = 0.99) with Wernicke’s to putamen greatest (0.109) followed by Broca’s to Wernicke’s (0.103) and Broca’s to putamen (0.095). gFA was larger in the left hemisphere (0.103) than the right (0.101; (F(1,70) =6.64, P = .012), but this hemispheric asymmetry was not the same for all tracts (Tract × Hemisphere interaction F(2,140) =3.33, P = .04, ε = 0.99). Subsequently rmANOVAs were performed for each tract.
Arcuate Fasciculus gFA
There were no significant differences in gFA between FEP and HC for bilateral arcuate fasciculus fibers (F(1,70) < 0.001, P = .994), and no asymmetry between hemispheres in either group (F(1,70) = 0.008, P = .930, table 4). However, among FEP left arcuate fasciculus gFA correlated robustly with hallucination severity (ρ = −0.41, P = .009, figure 2B, table 5), but right hemisphere arcuate fasciculus gFA did not attain significance (ρ = −0.244, P = .141). The arcuate fasciculus-AH association strength was not significantly different between hemispheres using a within-subjects z-transform comparison (P = .144), but would attain significant difference with a sample size of 90.
Broca’s Area to Putamen gFA
For bilateral Broca’s area to putamen fibers, there were no significant differences in gFA between FEP and HC (F(1,70) = 2.693, P = .105), and no asymmetry between hemispheres in either group (F(1,70) = 2.525, P = .117, table 4). Among FEP, neither left nor right Broca’s area to putamen gFA correlated significantly with hallucination severity (table 5).
Wernicke’s Area to Putamen gFA
For bilateral Wernicke’s area to putamen fibers, there were no significant differences in gFA between FEP and HC (F(1,64) =1.955, P = .166). The left hemisphere Wernicke’s area to putamen fibers showed greater gFA than the right hemisphere fibers (F(1,70) =7.367, P = .008), and this left greater than right asymmetry did not differ between FEP and HC (F(1,70) =1.221, P = .273, table 4). Among FEP, neither left nor right Wernicke’s area to putamen gFA correlated significantly with hallucination severity (table 5).
Discussion
FEP did not show overall white matter deficits in the tracts studied. However, gFA in the interhemispheric auditory connections was robustly associated AH severity, and among overall weak correlations in bilateral tracts, the left arcuate fasciculus showed the most robust association. Together, these results suggest early microstructural white matter abnormalities in psychosis that may relate to symptoms, yet be too small to detect as overall group differences. We show for the first time that, despite group gFA in the normal range, AH are associated with impaired white matter integrity in FEP. Although displaying only subtle microstructural white matter abnormalities, the major frontal-temporal left hemisphere language-related connection and auditory cortex interhemispheric communication were robustly associated with hallucination severity in FEP. Although the associations in the Hoffman hallucination circuit were most robust in the left arcuate fasciculus, all of the remaining bilateral tracts showed some degree of inverse relationship between gFA and AH, albeit nonsignificantly (table 5). Thus, the present data cannot determine if this white matter integrity—psychotic symptom association is specific to the left arcuate fasciculus or reflects a more widespread abnormality. To the degree that reduced directionality in fiber tracts results in slowed conduction times,26 this finding suggests mistiming of information flow in language/semantic circuits and between hemispheres in both primary and secondary auditory cortices.
Symptom capture studies have identified several nodes that appear to be functional hubs in AH17,54 including Broca’s area, Wernicke’s area, and the putamen. Using this framework to generate a priori hypotheses of dysconnectivity between the nodes in AH, we found left Broca’s area to putamen and right Wernicke’s to putamen gFA reductions in AH+ FEP relative to controls. We also observed reduced gFA in auditory transcallosal connections in AH+ relative to AH-. Interestingly, the connections to putamen are unidirectional, suggesting a major impairment of auditory-related cortical information flow to the basal ganglia in AH+ (Cortical projections from striatal areas arise from the globus pallidus). While it remains unknown precisely what role the putamen plays in AH, reduced volume,65 abnormal hemodynamic coupling,17 and now dysconnection with Broca’s and Wernicke’s areas appear to reflect pathology specific to AH. Thus, as proposed by Hoffman et al,17 abnormal putamen connectivity may play a role in abnormal activations of semantic stores in concert with dys-coordination between Broca’s and Wernicke’s areas. However, the overall gFA reductions in AH+ must be interpreted with caution due to the lack of an overall group difference in the omnibus ANOVA, and need replication and validation.
Several other caveats need to be mentioned. The hypothesis-driven analyses may overlook white matter bundles outside the tracts-of-interest that are associated with AH. For example, one major cortical area implicated in several fMRI studies of AH is the parahippocampal gyrus (refs.66–68, see also meta-analysis69 and review64). The hippocampus and its outputs through thalamus to medial prefrontal cortex also likely contribute to the phenomenology of AH.69–71 Whole-brain analyses with discovery and replication samples are needed to determine a data-driven tractography of AH, studies underway in our laboratory and others. The gray matter seed areas are based on relatively gross Brodmann’s areas. For example, we used BA 22 as a proxy for Wernicke’s. A more precise parcellation would better isolate the tracts-of-interest. We are currently developing a pipeline to utilize the HCP quasi-functional cortical parcellation scheme for white matter tractography. An atlas of pre-defined white matter tracts not relying on gray matter parcellation would be optimal and several are in development. We used gFA, the DSI analog of DTI FA, in this study for consistency with previous studies. Future work will more fully utilize the metrics specific to DSI that may better characterize the microstructural deficits in the tracts-of-interest. In the spirit of a trans-diagnostic systems neuroscience (RDoC) approach, we examined a specific symptom (AH) in FEP without regard to the primary diagnosis. Still, affective psychosis was relatively under-represented. Future work should expand the subject Ns available to probe the symptom-specific (AH+ vs AH-) vs diagnosis-specific (Sz spectrum vs affective psychosis) nature of these deficits.
In summary, we showed associations between AH severity and white matter integrity in FEP. Even at first clinical contact for psychosis, white matter dysconnectivity appears to be associated with AH. Understanding the gray and white matter structural deficits involved in AH may allow for the development of novel therapeutic interventions, such as precisely targeted noninvasive brain stimulation to normalize underlying neural pathophysiology.
Acknowledgments
We thank the faculty and staff of the WPH Psychosis Recruitment and Assessment Center for their assistance in recruitment, diagnostic and psychopathological assessments, and neuropsychological evaluations. We thank Marek Kubicki, Sylvain Bouix, and Martha E Shenton for helpful technical advice, and James Levitt for thoughtful discussion of the unidirectional cortical input to the putamen. Portions of this work formed the Senior Undergraduate Thesis of Y.W. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Funding
National Institutes of Health (R01 MH113533 to D.F.S. [PI] and P50 MH103204 to D.F.S. [Project Co-PI]).
References
- 1. Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15(9):5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binder JR, Frost JA, Hammeke TA, Rao SM, Cox RW. Function of the left planum temporale in auditory and linguistic processing. Brain. 1996;119 (Pt 4):1239–1247. [DOI] [PubMed] [Google Scholar]
- 3. Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cereb Cortex. 1996;6(1):11–20. [DOI] [PubMed] [Google Scholar]
- 4. Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10(1):15–35. [DOI] [PubMed] [Google Scholar]
- 5. Birn RM, Kenworthy L, Case L, et al. . Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage. 2010;49(1):1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27(10):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31(2):329–338. [DOI] [PubMed] [Google Scholar]
- 8. Schlösser R, Hutchinson M, Joseffer S, et al. . Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry. 1998;64(4):492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuperberg GR, Deckersbach T, Holt DJ, Goff D, West WC. Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Arch Gen Psychiatry. 2007;64(2):138–151. [DOI] [PubMed] [Google Scholar]
- 10. Crossley NA, Mechelli A, Fusar-Poli P, et al. . Superior temporal lobe dysfunction and frontotemporal dysconnectivity in subjects at risk of psychosis and in first-episode psychosis. Hum Brain Mapp. 2009;30(12):4129–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabb FW, van Erp TG, Hardt ME, et al. . Language network dysfunction as a predictor of outcome in youth at clinical high risk for psychosis. Schizophr Res. 2010;116(2-3):173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dierks T, Linden DE, Jandl M, et al. . Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22(3):615–621. [DOI] [PubMed] [Google Scholar]
- 13. Lennox BR, Park SBG, Medley I, et al. . The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Research: Neuroimaging. 2000;100(1):13–20. [DOI] [PubMed] [Google Scholar]
- 14. Strik W, Dierks T, Hubl D, Horn H. Hallucinations, thought disorders, and the language domain in schizophrenia. Clin EEG Neurosci. 2008;39(2):91–94. [DOI] [PubMed] [Google Scholar]
- 15. Wolf AW, Schubert DS, Patterson MB, Grande TP, Brocco KJ, Pendleton L. Associations among major psychiatric diagnoses. J Consult Clin Psychol. 1988;56(2):292–294. [DOI] [PubMed] [Google Scholar]
- 16. Kindler J, Homan P, Jann K, et al. . Reduced neuronal activity in language-related regions after transcranial magnetic stimulation therapy for auditory verbal hallucinations. Biol Psychiatry. 2013;73(6):518–524. [DOI] [PubMed] [Google Scholar]
- 17. Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Curcic-Blake B, Liemburg E, Vercammen A, et al. . When Broca goes uninformed: reduced information flow to Broca’s area in schizophrenia patients with auditory hallucinations. Schizophr Bull. 2013;39(5):1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suzuki M, Yuasa S, Minabe Y, Murata M, Kurachi M. Left superior temporal blood flow increases in schizophrenic and schizophreniform patients with auditory hallucination: a longitudinal case study using 123I-IMP SPECT. Eur Arch Psychiatry Clin Neurosci. 1993;242(5):257–261. [DOI] [PubMed] [Google Scholar]
- 20. Hoffman RE, Boutros NN, Hu S, Berman RM, Krystal JH, Charney DS. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet. 2000;355(9209):1073–1075. [DOI] [PubMed] [Google Scholar]
- 21. Hoffman RE, Hawkins KA, Gueorguieva R, et al. . Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60(1):49–56. [DOI] [PubMed] [Google Scholar]
- 22. Hoffman RE, Hampson M, Wu K, et al. . Probing the pathophysiology of auditory/verbal hallucinations by combining functional magnetic resonance imaging and transcranial magnetic stimulation. Cereb Cortex. 2007;17(11):2733–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hubl D, Koenig T, Strik W, et al. (2004). Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 61(7), 658–668. [DOI] [PubMed] [Google Scholar]
- 24. Ford JM, Mathalon DH. Corollary discharge dysfunction in schizophrenia: can it explain auditory hallucinations? Int J Psychophysiol. 2005;58(2-3):179–189. [DOI] [PubMed] [Google Scholar]
- 25. Ford JM, Mathalon DH, Roach BJ, et al. . Neurophysiological evidence of corollary discharge function during vocalization in psychotic patients and their nonpsychotic first-degree relatives. Schizophr Bull. 2013;39(6):1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whitford TJ, Mathalon DH, Shenton ME, et al. . Electrophysiological and diffusion tensor imaging evidence of delayed corollary discharges in patients with schizophrenia. Psychol Med. 2011;41(5):959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abdul-Rahman MF, Qiu A, Woon PS, Kuswanto C, Collinson SL, Sim K. Arcuate fasciculus abnormalities and their relationship with psychotic symptoms in schizophrenia. PLoS One. 2012;7(1):e29315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whitford TJ, Oestreich LKL, Ford JM, et al. . Deficits in cortical suppression during vocalization are associated with structural abnormalities in the arcuate fasciculus in early illness schizophrenia and clinical high risk for psychosis. Schizophr Bull. 2018;44(6):1312–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boos HB, Mandl RC, van Haren NE, et al. . Tract-based diffusion tensor imaging in patients with schizophrenia and their non-psychotic siblings. Eur Neuropsychopharmacol. 2013;23(4):295–304. [DOI] [PubMed] [Google Scholar]
- 30. Burns J, Job D, Bastin ME, et al. . Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- 31. Kubicki M, Park H, Westin CF, et al. . DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Catani M, Craig MC, Forkel SJ, et al. . Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol Psychiatry. 2011;70(12):1143–1150. [DOI] [PubMed] [Google Scholar]
- 33. de Weijer AD, Mandl RC, Diederen KM, et al. . Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res. 2011;130(1-3):68–77. [DOI] [PubMed] [Google Scholar]
- 34. de Weijer AD, Neggers SF, Diederen KM, et al. . Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non-psychotic individuals. Hum Brain Mapp. 2013;34(3):626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCarthy-Jones S, Oestreich LK, Whitford TJ; Australian Schizophrenia Research Bank Reduced integrity of the left arcuate fasciculus is specifically associated with auditory verbal hallucinations in schizophrenia. Schizophr Res. 2015;162(1-3):1–6. [DOI] [PubMed] [Google Scholar]
- 36. Phillips OR, Nuechterlein KH, Clark KA, et al. . Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr Res. 2009;107(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, et al. . Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Res. 2009;174(1):9–16. [DOI] [PubMed] [Google Scholar]
- 38. Seok JH, Park HJ, Chun JW, et al. . White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Res. 2007;156(2):93–104. [DOI] [PubMed] [Google Scholar]
- 39. Shergill SS, Kanaan RA, Chitnis XA, et al. . A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164(3): 467–473. [DOI] [PubMed] [Google Scholar]
- 40. Pasternak O, Westin CF, Bouix S, et al. . Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32(48):17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheung V, Cheung C, McAlonan GM, et al. . A diffusion tensor imaging study of structural dysconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2008;38(6):877–885. [DOI] [PubMed] [Google Scholar]
- 42. Peters BD, de Haan L, Dekker N, et al. . White matter fibertracking in first-episode schizophrenia, schizoaffective patients and subjects at ultra-high risk of psychosis. Neuropsychobiology. 2008;58(1):19–28. [DOI] [PubMed] [Google Scholar]
- 43. Cheung V, Chiu CP, Law CW, et al. . Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychol Med. 2011;41(8):1709–1719. [DOI] [PubMed] [Google Scholar]
- 44. Douaud G, Smith S, Jenkinson M, et al. . Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130(Pt 9):2375–2386. [DOI] [PubMed] [Google Scholar]
- 45. Szeszko PR, Ardekani BA, Ashtari M, et al. . White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry. 2005;162(3):602–605. [DOI] [PubMed] [Google Scholar]
- 46. Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol Psychiatry. 2008;63(5):512–518. [DOI] [PubMed] [Google Scholar]
- 47. Mandl RC, Rais M, van Baal GC, et al. . Altered white matter connectivity in never-medicated patients with schizophrenia. Hum Brain Mapp. 2013;34(9):2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40(4):721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cropley VL, Klauser P, Lenroot RK, et al. . Accelerated gray and white matter deterioration with age in schizophrenia. Am J Psychiatry. 2016;174(3):286–295. [DOI] [PubMed] [Google Scholar]
- 50. Viñas-Guasch N, Wu YJ. The role of the putamen in language: a meta-analytic connectivity modeling study. Brain Struct Funct. 2017;222(9):3991–4004. [DOI] [PubMed] [Google Scholar]
- 51. Gil Robles S, Gatignol P, Capelle L, Mitchell MC, Duffau H. The role of dominant striatum in language: a study using intraoperative electrical stimulations. J Neurol Neurosurg Psychiatry. 2005;76(7):940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Friederici AD, Rüschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13(2):170–177. [DOI] [PubMed] [Google Scholar]
- 53. Davies RR, Halliday GM, Xuereb JH, Kril JJ, Hodges JR. The neural basis of semantic memory: evidence from semantic dementia. Neurobiol Aging. 2009;30(12):2043–2052. [DOI] [PubMed] [Google Scholar]
- 54. Scheinost D, Tokoglu F, Hampson M, Hoffman R, Constable RT. Data-Driven analysis of functional connectivity reveals a potential auditory verbal hallucination network. Schizophr Bull. 2019;45(2):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cui LB, Liu K, Li C, et al. . Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173(1-2):13–22. [DOI] [PubMed] [Google Scholar]
- 56. Dandash O, Fornito A, Lee J, et al. . Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 2014;40(4):904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quan M, Lee SH, Kubicki M, et al. . White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res. 2013;145(1-3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bloemen OJ, de Koning MB, Schmitz N, et al. . White-matter markers for psychosis in a prospective ultra-high-risk cohort. Psychol Med. 2010;40(8):1297–1304. [DOI] [PubMed] [Google Scholar]
- 59. Mulert C, Kirsch V, Whitford TJ, et al. . Hearing voices: a role of interhemispheric auditory connectivity? World J Biol Psychiatry. 2012;13(2):153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ćurčić-Blake B, Nanetti L, van der Meer L, et al. . Not on speaking terms: hallucinations and structural network disconnectivity in schizophrenia. Brain Struct Funct. 2015;220(1):407–418. [DOI] [PubMed] [Google Scholar]
- 61. Samartzis L, Dima D, Fusar-Poli P, Kyriakopoulos M. White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. J Neuroimaging. 2014;24(2):101–110. [DOI] [PubMed] [Google Scholar]
- 62. Iraji A, Chen H, Wiseman N, et al. . Compensation through Functional Hyperconnectivity: A Longitudinal Connectome Assessment of Mild Traumatic Brain Injury. Neural Plast. 2016;2016:4072402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yeh FC, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng WY. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One. 2013;8(11):e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bohlken MM, Hugdahl K, Sommer IE. Auditory verbal hallucinations: neuroimaging and treatment. Psychol Med. 2017;47(2):199–208. [DOI] [PubMed] [Google Scholar]
- 65. van Tol MJ, van der Meer L, Bruggeman R, Modinos G, Knegtering H, Aleman A. Voxel-based gray and white matter morphometry correlates of hallucinations in schizophrenia: the superior temporal gyrus does not stand alone. Neuroimage Clin. 2014;4:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Silbersweig DA, Stern E, Frith C, et al. . A functional neuroanatomy of hallucinations in schizophrenia. Nature. 1995;378(6553):176–179. [DOI] [PubMed] [Google Scholar]
- 67. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57(11):1033–1038. [DOI] [PubMed] [Google Scholar]
- 68. Escartí MJ, de la Iglesia-Vayá M, Martí-Bonmatí L, et al. . Increased amygdala and parahippocampal gyrus activation in schizophrenic patients with auditory hallucinations: an fMRI study using independent component analysis. Schizophr Res. 2010;117(1):31–41. [DOI] [PubMed] [Google Scholar]
- 69. Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2016;69:113–123. [DOI] [PubMed] [Google Scholar]
- 70. Stephane M. Auditory verbal hallucinations result from combinatoric associations of multiple neural events. Front Hum Neurosci. 2013;7:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stephane M, Thuras P, Nasrallah H, Georgopoulos AP. The internal structure of the phenomenology of auditory verbal hallucinations. Schizophr Res. 2003;61(2-3):185–193. [DOI] [PubMed] [Google Scholar]