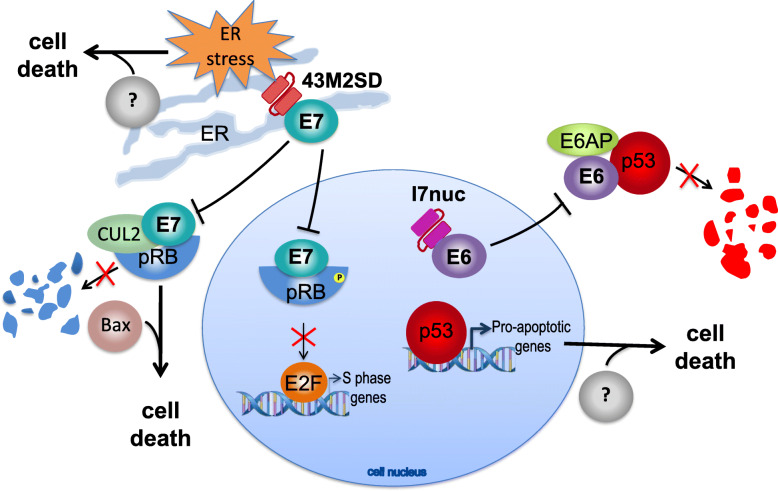
Fig. 5.
Hypothetical mechanisms of induction of apoptosis by the anti-16E6 and anti-16E7 intrabodies. The binding of scFvI7nuc (I7nuc) to E6 can inhibit the cytoplasmic degradation of p53. The restored levels of nuclear p53 may activate the transcription of pro-apoptotic genes including Puma, Noxa, Bak and Bax and the subsequent loss of mitochondrial membrane potential, with downstream activation of the executor caspase 3, finally leading to cell death. The binding of scFv43M2SD (43M2SD) to E7 can inhibit its translocation to nucleus and the subsequent inactivation of the Retinoblastoma tumor suppressor (pRB) thus restoring the control of E2F transcription factors by pRb. At the same time, the binding of scFv43M2SD can inhibit the association of E7 with the cullin 2 complex (CUL2) and the recruitment of pRB for ubiquitination. Increased levels of pRB, regardless of its role as a transcriptional regulator, can directly activate the BAX apoptosis regulator at mitochondrial level and promote cell death [34]. The binding of scFv43M2SD to KDEL receptors of ER may also induce ER stress-related molecules which can cause apoptosis by triggering the activation of caspases