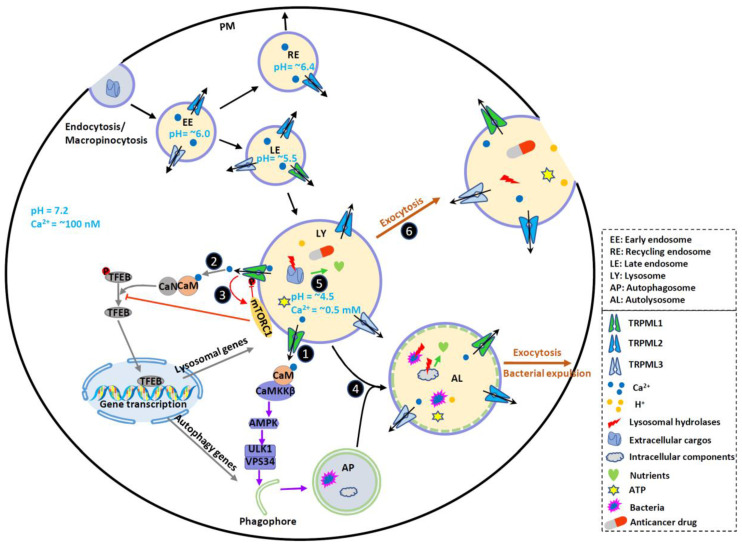
Figure 1.
TRPMLs in endocytic, phagocytic and autophagic pathways and tumor progression. TRPMLs are predominately located on the endolysosomal pathway, and they have been involved in endocytic, phagocytic and autophagic pathways. In the tumor microenvironment, autophagy is activated to help cancer cells digest damaged or nonessential proteins and organelles, meeting the increased energy and nutrient demand of cancer cells. By releasing intraluminal Ca2+, TRPMLs have been implicated in intracellular Ca2+ signaling, endolysosome trafficking, and lysosomal functions, further regulating autophagy. First, TRPML1 activates calmodulin (CaM)/CaMKKβ/AMPK pathway to promote autophagosome formation. Second, TRPML1 activates CaM/CaN/TFEB pathway to continuously supply lysosome and autophagy proteins. Third, TRPML1 maintains mTORC1 activity to prevent cancer cell death and promote lysosome reformation. In normal fed conditions, mTORC1 phosphorylates TRPML1 at S571 and S576 to inhibit its activity. Starvation reduces mTORC1 activity and disinhibits TRPML1. This subsequently promotes mTORC1 activity, preventing cell death. Fourth, TRPML1 stimulates Apoptosis-linked gene-2 (ALG-2)-dependent lysosome centripetal movement to facilitate autophagosome-lysosome fusion. Fifth, TRPML1 increases lysosomal degradative functions, likely through controlling lysosomal pH. Sixth, TRPML1 increases Syt7-dependent lysosomal exocytosis, releasing hydrolases, ATP and H+ to extracellular spaces. These may change tumor microenvironment, promote ECM degradation, and facilitate tumor progression. By promoting lysosomal exocytosis, TRPML1 may also participate in drug resistance by releasing sequestrated anticancer drugs. Due to the functional redundancy between the TRPML proteins, TRPML2 and TRPML3 may also contribute to some of these events to regulate cancer development. Therefore, TRPMLs orchestrate all these cellular events to help cancer cells maintain high autophagic flux and adapt to the tumor microenvironment.