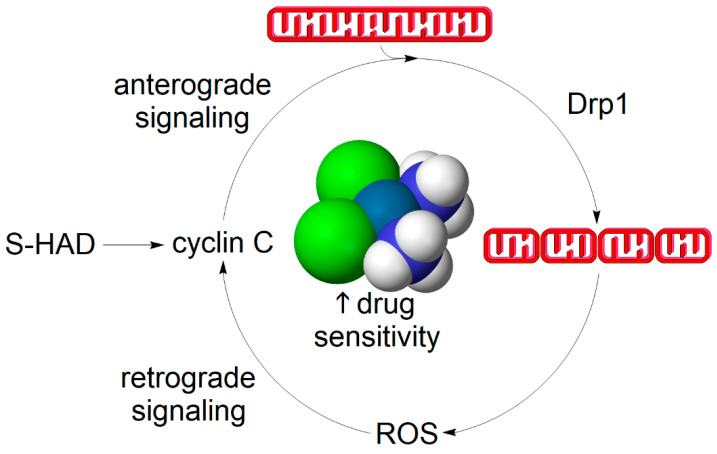
Figure 4.
A hypothetical self-propelling cycle between mitochondrial fission and ROS generation leading to the mitochondrial fragmentation phenotype. Cyclin C association with its nuclear anchor Med13 is disrupted by treatment with the stapled peptide mimetic of the N-terminal domain of cyclin C (S-HAD) or upon exogenous oxidative stress such as H2O2 or cisplatin treatment. Cyclin C is partially released from the nucleus to bind and activate Drp1 and thereby induce mitochondrial fission. Mitochondrial fragments are more likely to emanate ROS signals such as in the form of H2O2 derived from mitochondrially-produced superoxide. This could be either due to mitochondrial dysfunction or a transient rise in respiratory function. Nevertheless, mitochondrial ROS-mediated retrograde signaling may act on cyclin C through an unknown redox sensor. The closing of the positive feedback loop between mitochondrial fission and mitochondrial ROS formation contributes to rapid amplification of the redox signal, also referred to as ROS burst. Advantageously, tipping ROS levels over apoptosis-inducing threshold through generating an ROS burst, such as using S-HAD, renders cancer cells more sensitive to chemotherapeutic interventions [87]. Other signaling pathways may participate in relaying the redox signal to the mitochondrial fission machinery as well and mitochondrially-derived ROS may short-circuit this cycle by directly modulating Drp1 activity [3].