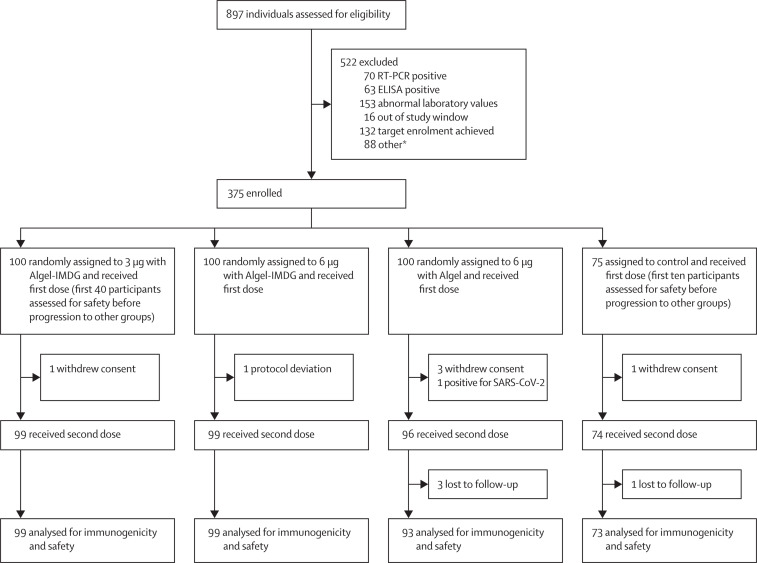
Figure 1.
Trial profile
Due to a sudden increase in interest from potential participants, parallel enrolment at several sites, and an oversight in the trial software, 27 of the 132 people who reached target enrolment after the planned size of 375 people was reached received a first dose and 26 of them a second dose. They were not included in further safety or immunogenicity trial data, but were monitored for safety with 3 people reporting injection site pain and 1 person a tingling sensation after the first dose, and 1 person drowsiness, 2 people headache, and 1 person injection site pain after the second dose. No serious adverse events occurred. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Unable to contact the participant for vaccination or withdrawal of consent.