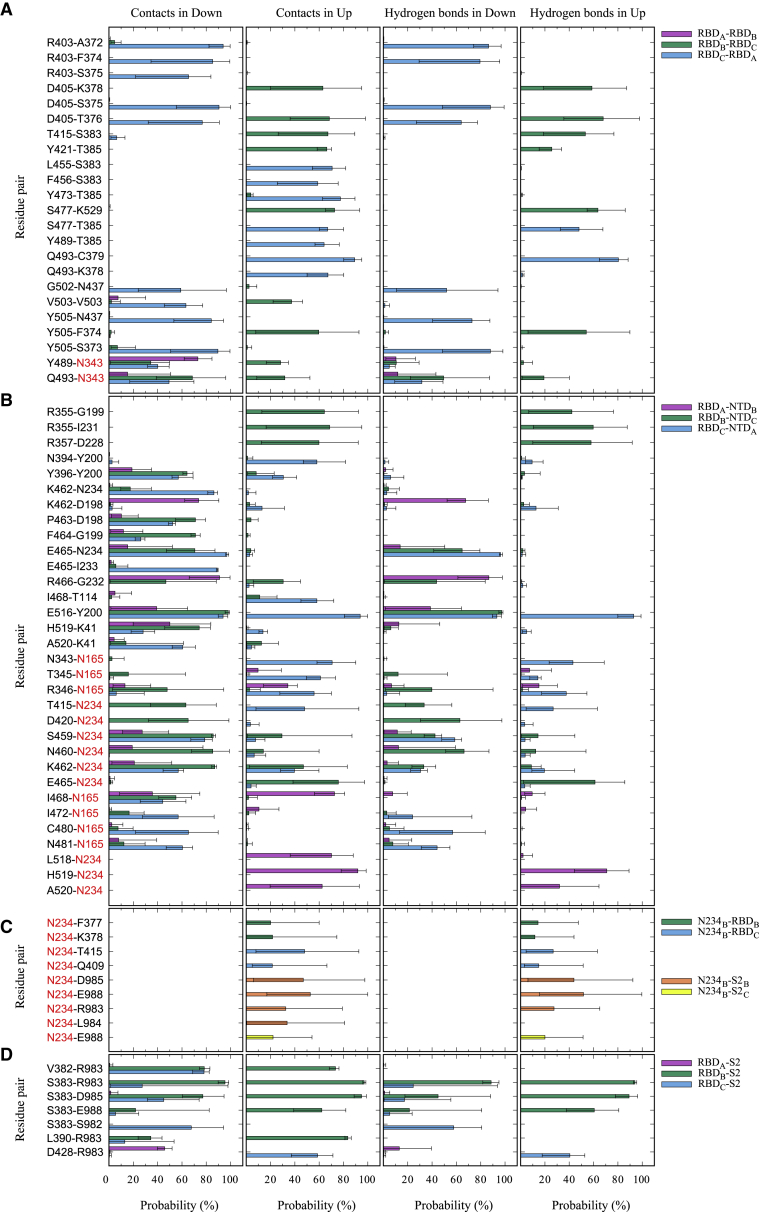
Figure 3.
The interdomain contacts and hydrogen bonds in MD1_Down and MD1_Up. (A) The interactions between different RBDs, RBDA-RBDB (purple), RBDB-RBDC (green), and RBDC-RBDA (light blue); (B) those between RBDs and NTDs, RBDA-NTDB (purple), RBDB-NTDC (green), and RBDC-NTDA (light blue); (C) those between N234 and RBDs/S2, N234B-RBDB (green), N234B-RBDC (light blue), N234B-S2B (orange), and N234B-S2C (yellow); and (D) those between RBDs and S2, RBDA-S2 (purple), RBDB-S2 (green), and RBDC-S2 (light blue), are shown. Red characters mean the glycosylated amino acid residues. In the analysis, a contact is defined when the minimal distance between two residues is shorter than 2.5 Å. A hydrogen bond is decided if the D…A (donor…acceptor) distance is shorter than 3.4 Å, the D-H…A angle is smaller than 120°, and the H-D…A angle is greater than 30°. The last 500-ns trajectory is divided into five 100-ns trajectories, and the average numbers of contacts and hydrogen bonds are shown as bars. The maximal and minimal numbers are shown in error bars. Only the residue pairs that have more than 55% (for A, B, and D) or 20% (for C) of contacts in either MD1_Down or MD1_Up are shown. To see this figure in color, go online.