Abstract
In December 2020, the US Food and Drug Administration issued emergency use authorizations for two mRNA vaccines against coronavirus disease 2019. These vaccines represent an incredible scientific achievement and a major step in efforts to bring the global pandemic to a close. However, these vaccines create many logistical challenges that limit just how far-reaching their impact can be. This commentary reviews how these vaccines offer immunity, summarizes the Phase III trial results, and offers a discussion of the challenges that remain after these vaccines are introduced for widespread use.
Key words: COVID-19, mRNA vaccines, SARS CoV-2, Spike protein
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has inflicted a heavy toll on the domestic and global population, with nearly 7% of the total US population having evidence of infection and >80 million cases worldwide at the time of writing.1 With the recent emergency use authorization (EUA) of two mRNA vaccine candidates (Moderna [Cambridge, Massachusetts] and Pfizer [New York, New York]/BioNTech [Mainz, Germany]) by the US Food and Drug Administration, there is widespread hope of an inflection point in this pandemic that shifts from consecutive waves of increased cases to a gradual blunting of transmission that eventually leads to long-term control.2 , 3 This commentary offers a brief summary of the preclinical and clinical development of the mRNA vaccines and a perspective on how these fit into our global public health goals for bringing this pandemic to a close.
Brief Review of the COVID-19 Timeline
Widespread attention to cases of an unknown respiratory illness in Wuhan, China, began in December 2019, with subsequent identification of the cause as a Betacoronavirus related to the severe acute respiratory syndrome coronavirus (SARS-CoV) source of the global 2003 outbreak.4 Within 1 month, a full-length sequence of the dubbed SARS-CoV-2 (SARS-CoV-2) was available for global dissemination so researchers could begin to address the challenges of immunity. Within 5 days of the sequence being distributed, an mRNA vaccine candidate was produced using Good Manufacturing Practices for early testing.5 Within 66 days, a Phase I human study was begun and just over 2 months later, a Phase II human study was initiated. The magnitude of this collaborative scientific achievement must be acknowledged.
Historically, vaccine development to combat other viruses has occurred at a much slower pace. A vaccine candidate was not developed in 2003 with the initial SARS-CoV outbreak. With transmission localized primarily to 5 countries, suppression was achieved by contact tracing and isolation. Vaccine candidates for the 2009 influenza A(H1N1) pandemic influenza strain were available for expedited testing in ~4 months after sequencing, capitalizing on the infrastructure for seasonal vaccine production.6 , 7 Full-scale production and distribution occurred in an expedited process after an additional 6 months in November 2009. In contrast, application of the mRNA platforms for successful vaccine delivery was novel. Proof-of-concept studies using mRNA platforms to rapidly develop possible vaccine candidates for pandemic influenza strains supported the idea that these could be produced in rapid response to a global outbreak.8, 9, 10
Despite almost 3 decades of exploration with this technology, the discussions had by public and private partners about how mRNA vaccines could be optimized to address a global pandemic helped move things forward. With entirely new safety and efficacy data in an environment of significant anti-science sentiment and vaccine hesitancy, trust and confidence were paramount to achieving the EUA.
mRNA Vaccine–Induced Immunity Versus Natural Immunity
To understand how the mRNA vaccines work, it is helpful to review a few key facts about the steps involved in viral infection and the subsequent immune response. First, mRNA is a natural set of instructions essential to the reproduction and life cycle of any virus, serving as a code for the proteins required for viral replication or assembly of new viral particles. The SARS-CoV-2 mRNA vaccines encode for the spike protein, which is a fusion protein required for viral entry.11 Prior studies evaluating immunity to SARS-CoV and Middle East respiratory syndrome–related coronavirus showed that immunity to the spike protein was protective of clinical infection.12 Second, when a virus enters the host cell, fusion proteins undergo a key conformational change that facilitates the entry of genetic material to being the replication process.13 Immunity to the “prefusion” conformation is protective to the host, but immunity to the postfusion conformation offers no protection and may even be responsible for a dysregulated immune response.12 A key step in the production of the mRNA vaccines was to introduce 2 proline substitutions to “lock” the spike protein in a prefusion conformation and prevent change to the postfusion state so vaccination would offer the appropriate protection.5
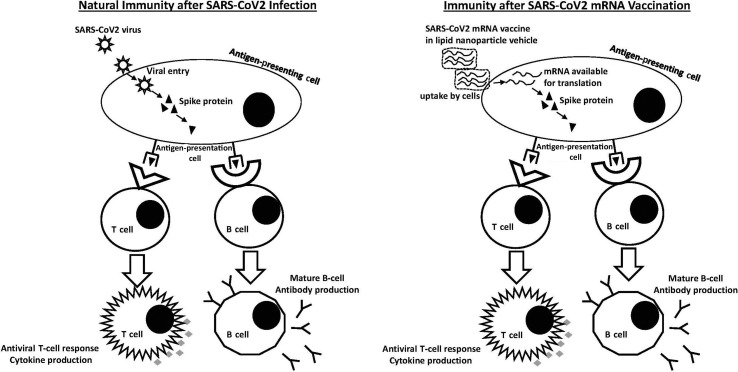
During natural infection, virus enters cells and produces the spike protein. These foreign proteins are recognized by antigen-presenting cells and are used to activate T and B cells to general specific antibodies to protect against subsequent infection (Figure 1 ). In contrast, mRNA vaccines directly introduce the genetic material to code to spike protein in antigen-presenting cells. The protein is produced and presented in the same way as natural infection. It is important to emphasize the unknowns that come with these vaccines. Currently approved vaccines for human use include formalin-inactivated whole virus (eg, hepatitis A), live attenuated virus (eg, measles, mumps, and rubella), or recombinant subunit vaccines (eg, hepatitis B). It is unclear just how long mRNA vaccine protection will last, but in theory, once antiviral T cells and memory B cells are generated, immunity should be durable for many years.
Figure 1.
Natural immunity after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection versus immunity after SARS-CoV-2 mRNA vaccination. During viral infection, the SARS-CoV-2 virus is taken up by antigen-presenting cells. Individual proteins such as the spike protein are presented to naive T cells and B cells to stimulate specific antiviral T cells and memory B cells to protect against future infection. With mRNA vaccination, mRNA is taken up by cells via the lipid nanoparticle vehicle and is then translated in the cytoplasm to produce spike protein. This spike protein is then presented by antigen-presenting cells in the same way as during viral infection with the same downstream production of antiviral T cells and memory B cells.
Results of Early-Phase Clinical Trials
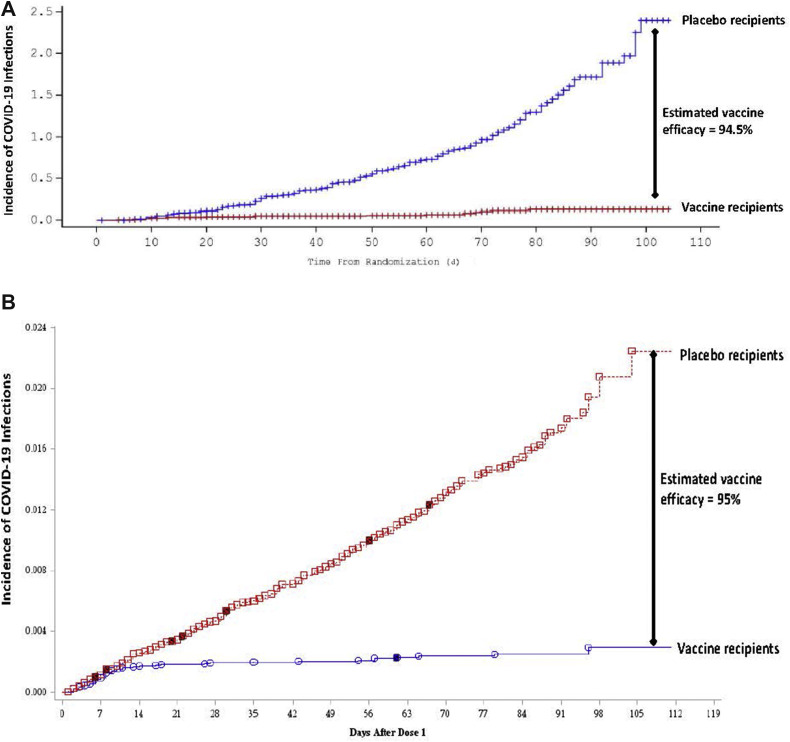
Within 2 months of sequence identification, a Phase I human study was conducted with the Moderna vaccine. Fifteen participants were given 2 vaccine doses of 25 μg, 100 μg, and 250 μg separated by 28 days.14 In short, the 100-μg dose offered the best combination of neutralizing antibody titers with acceptable reactogenicity. Doses of 50 μg and 100 μg were used for the subsequent Phase II study of 600 participants before progressing to the larger Phase III trial.15 In this study that formed the basis of the EUA, >30,000 participants were enrolled in a placebo-controlled trial. Randomized and blinded participants were given either 2 doses of either vaccine or saline injection, separated by 28 days, and followed up for reactogenicity and clinical symptoms consistent with COVID-19. An interim follow-up period of 2 months was planned to evaluate for safety and efficacy. Given the racial and ethnic disparities noted in patients affected by COVID-19, it is important to highlight that Black subjects comprised 10% of study participants, and 20% self-identified as Latino.16 By now, the interim results of this study have been well publicized (Figure 2 A). Vaccine efficacy was estimated to be 94.5% in all participants, which was consistent between those aged 18 and 65 years and those >65 years of age.
Figure 2.
Kaplan–Meier graphs illustrating vaccine efficacy with mRNA vaccines. Results of each of the Phase III trials with the mRNA vaccines are represented in these Kaplan–Meier plots showing incidence of new coronavirus disease 2019 (COVID-19) infections on the y-axis against days since randomization on the x-axis. (A) Results of the Phase III Moderna trial, with COVID-19 infections reaching 2.5% in the placebo group compared with 0.1% in the vaccinated group. This equates to an estimated vaccine efficacy of 94.5%. (Adapted from Moderna, Inc.15) (B) Results of the Phase III Pfizer/BioNTech trial, with COVID-19 infections reaching 2.4% in the placebo group compared with 0.03% in the vaccinated group. This equates to an estimated vaccine efficacy of 95%. (Adapted from Pfizer/BioNTech.18).
The Pfizer/BioNTech vaccine followed a similar path of development. Rodent and non-human primate studies showed acceptable immunogenicity, which was duplicated in human studies. A combined Phase I/II study was conducted with 45 participants randomized into 3 dose groups, 12 receiving doses of 10 μg, 30 μg, or 100 μg, and 3 participants in each group receiving placebo.17 Local reactogenicity was common in all dosing groups, and the 30-μg dose offered the best combination of immunogenicity and tolerable reactogenicity. The 30-μg dose was used in the large Phase III study, with results now well publicized.18 More than 37,000 randomized and blinded participants received 2 doses of either 30 μg of vaccine or saline injection, administered 21 days apart. The racial and ethnic mix of participants in this trial was similar to that of the Moderna trial, and the efficacy was similar as well. The interim evaluation was planned to occur after ninety-four COVID-19 cases were identified among all participants. Estimated vaccine efficacy was noted to be 95%, with 4 cases found in the vaccine group and 90 in the placebo group. A more complete evaluation was presented to the US Food and Drug Administration (FDA) before issuing of the EUA, with 170 total COVID-19 cases continuing to exhibit the same 95% estimated efficacy (Figure 2B). Efficacy among the cases identified in the vaccine group after 1 dose of vaccine resulted in an estimated efficacy against COVID-19 of 52% after 1 dose of the Pfizer/BioNTech vaccine.
Although the efficacy of the 2 mRNA vaccines is very similar, the major difference lies in the product storage requirements and temperature stability. The Pfizer/BioNTech mRNA vaccine requires storage at −60° to −80 °C, which requires either dry ice temporary storage or use of an “ultra-low” freezer.18 After thawing, the vaccine is diluted in saline and should be administered within 6 h. The requirement for either dry ice or ultra-low freezers severely limits the types of facilities where the cold chain can be maintained and the Pfizer/BioNTech vaccine administered. The Moderna mRNA vaccine is a bit more forgiving in its requirements. Long-term storage can be between −15° and −25 °C, the temperature of a conventional freezer, and after thawing can be kept at 2° to 8 °C for 30 days or room temperature if unopened for 12 h and once opened discarded after 6 h.15 These more flexible storage requirements have allowed the Moderna product to be distributed to health departments, urgent care centers, clinical practices, and most importantly to rural entities where essential workers and residents of long-term care facilities may be immunized.
Many have expressed concern at the speed of approval for both vaccines and whether safety requirements were fulfilled appropriately. Although the EUA mechanism of approval does not convey licensure, it does facilitate use under a situation that is deemed to be emergent. Under the traditional vaccine approval process, the prolonged timeline is due to industry's hesitancy in investing in large-scale production without Phase II and III efficacy studies and an almost guarantee of FDA licensure. The US Department of Defense initiative titled "Operation Warp Speed" removed the financial risk of early large-scale production so this could take place coincident with Phase III studies. With >30,000 participants each, the size of the two Phase 3 trials for these vaccines is comparable to the size of other Phase III studies for currently licensed vaccines.19 , 20 The safety data generated from the Pfizer/BioNTech and Moderna Phase III studies are equivalent to the enrollments for traditional Phase III vaccine licensing requirements. However, ongoing monitoring for rare adverse events will need to be conducted under the terms of the EUA and as traditional licensure is likely pursued.
What Comes Next? Is the Pandemic Over?
The development of these first vaccines is an incredible achievement and offers public health officials and the medical community cause for some optimism and another tool in a valued armamentarium against SARS-CoV-2. However, this optimism needs to be tempered with acknowledgement of many factors that may limit the impact of these mRNA vaccines.
As was already mentioned, the cold chain storage requirements for both products will restrict where and who can administer vaccines. The Pfizer/BioNTech vaccine lends itself to administration in hospitals and large health care facilities with ready access to ultra-low freezers or dry ice. The more flexible storage requirements of the Moderna vaccine have facilitated its use in public health and community settings to vaccinate the elderly and residents of congregate facilities. The considerable experience vaccinating adult and pediatric inpatients and specialty clinic outpatients with seasonal influenza and pneumococcal vaccines could be leveraged to do the same for COVID-19 vaccination of those receiving acute and subspecialty care in these settings.21 , 22 Workflows for immunizing children and adults who do not access the health care system regularly will need to be developed. There are opportunities for public–private partnerships with commercial pharmacies and educational facilities to increase uptake. Other vaccine candidates that require only 1 dose and can be stored without freezing will further facilitate expanded access.
Although there were important attempts to achieve racial and ethnic diversity in Phase III trial enrollment, several important subpopulations were not included in study enrollment. Pregnant women comprise a significant portion of the health care workforce who are among the highest priority for receiving vaccine. Despite the fact that no research was done to examine mRNA vaccine safety and efficacy in pregnant women, the Advisory Committee on Immunization Practices COVID-19 task force included pregnant health care workers, with certain cautions in their recommendations for early vaccination.23 Although children have made up ~10% of COVID-19 cases in the United States and abroad, children aged <16 years are excluded from receiving either of the mRNA vaccines under the EUA.24 Although children have comprised a smaller proportion of severely ill patients, they have been kept out of school for fear of the risk of spreading the virus to other children, teachers, and their own family members.25 Any long-term strategy for control of COVID-19 will need to include plans for universal vaccination of children with the involvement of pediatric health care providers.26
One also needs to consider many of the challenges to the logistics of trying to vaccinate most of the domestic and global population. Moderna has never had an approved vaccine before, and neither has BioNTech. Both have produced other mRNA products on a small scale but nowhere near the hundreds of millions of doses that will be required. It is not surprising that there have been shortfalls in the number of expected doses ready to be administered in the early weeks after the EUAs were granted.27
Our US system falls short with reliably delivering vaccines. Although routine childhood vaccination rates are >90%, these are given to an annual birth cohort of <4 million children.28 Seasonal influenza vaccine is recommended for everyone aged ≥6 months; however, even with public health education efforts to encourage people to get vaccinated, rates hover around 50%.29 The highest rate of seasonal influenza uptake, approaching 75%, in children aged <5 years is likely the result of receipt of routine child care in a medical home. A more sobering uptake of 38% in adults aged 18–49 years results from the absence of a medical home and receipt in an array of workplace, public health, and retail sources. Given the increased transmissibility of the SARS-CoV-2 virus, estimates have suggested that ~70%–80% of the population will need to be vaccinated to halt the spread of the virus.30 This will take several different vaccine products saturating every possible outlet for delivery plus new and creative ways to administer vaccines well beyond what has been done previously. This will also require addressing widespread apprehension and vaccine hesitancy among the general population and outreach to disadvantaged racial and ethnic minority groups.31 In the meantime, the need for universal masking, rigorous hand hygiene, and social distancing will need to remain in place as the primary means of ongoing prevention.
These mRNA vaccines offer little promise to prevent COVID-19 infections in resource-limited settings. Affluent countries have purchased advance access to hundreds of millions of doses as they are being produced for the next several years.32 Estimates are that supply would not be available until 2022 or beyond for global use. The cost of these vaccines prevents them from being an option for most global settings, and this is why the World Health Organization has set up a program called COVAX, which is designed to ensure that affordable COVID-19 vaccine candidates are secured for distribution in resource-limited settings.33 The vaccine candidate from AstraZeneca and Oxford that just received EUA in the United Kingdom has been targeted for resource-limited settings with an anticipated cost of $3 per dose.34 The specifics of this vaccine are distinct from the mRNA vaccines and are outside the scope of the current discussion. Although the estimated vaccine efficacy is only ~70%, it still offers a much-needed option for the United Kingdom and for the COVAX global initiative to deliver hope to resource-limited countries.35 The impact of the pandemic in these settings has been devastating, and thus access to a vaccine to help them manage the cases for the global population will be critical.
Conclusions
The two COVID-19 mRNA vaccines represent an incredible scientific achievement and offer hope to reduce the devastating impact that COVID-19 has had both domestically and internationally (Figure 3). Enthusiasm for their potential should be tempered by the operational aspects of uptake and delivery, all of which will be required to reduce the clinical and societal morbidity and mortality. Over the next several months and years, as more vaccine products receive regulatory approval and become available, the challenges of supply and need will hopefully be tempered along with the spread of SARS-CoV-2.
Figure 3.
Author sticker after receipt of the first dose of coronavirus disease 2019 (COVID-19) vaccine.
Disclosures
The author has indicated that he has no conflicts of interest regarding the content of this article. Dr. Jhaveri is Co-Editor-in-Chief of Clinical Therapeutics. He was not involved in peer review process for this article.
Acknowledgments
The author thanks Andi Shane, MD, MPH, for her thoughtful and detailed input on the content of this commentary, and Taylor Heald-Sargent, MD, PhD for her input on content of the figures. He offers his best wishes to health care worker colleagues who continue to work under challenging circumstances to provide care to those in need in this pandemic.
References
- 1.Johns Hopkins Coronavirus Resource Center . 2020. COVID-19 Global Cases Data Center.https://coronavirus.jhu.edu/data/new-cases Available from: [Google Scholar]
- 2.FDA takes key action in fight against COVID-19 By issuing emergency use authorization for first COVID-19 vaccine. US Food and Drug Administration. 2020 https://www.fda.gov/news-events/press-announcements/fda-takes-key-action-fight-against-covid-19-issuing-emergency-use-authorization-first-covid-19 [Google Scholar]
- 3.FDA takes additional action in fight against COVID-19 By issuing emergency use authorization for Second COVID-19 vaccine. US Food and Drug Administration. 2020 https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid [Google Scholar]
- 4.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corbett K.S., Edwards D.K., Leist S.R., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W.H., Winokur P.L., Edwards K.M., et al. Phase 2 assessment of the safety and immunogenicity of two inactivated pandemic monovalent H1N1 vaccines in adults as a component of the U.S. pandemic preparedness plan in 2009. Vaccine. 2012;30:4240–4248. doi: 10.1016/j.vaccine.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff K.L., Halasa N.B., Harrison C.J., et al. Clinical and immune responses to inactivated influenza A(H1N1)pdm09 vaccine in children. Pediatr Infect Dis J. 2014;33:865–871. doi: 10.1097/INF.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascola J.R., Fauci A.S. Novel vaccine technologies for the 21st century. Nat Rev Immunol. 2020;20:87–88. doi: 10.1038/s41577-019-0243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman R.A., Fuhr R., Smolenov I., et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 10.Pardi N., Parkhouse K., Kirkpatrick E., et al. Nucleoside-modified mRNA immunization elicits influenza virus hemagglutinin stalk-specific antibodies. Nat Commun. 2018;9:3361. doi: 10.1038/s41467-018-05482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heald-Sargent T., Gallagher T. Ready, set, fuse! the coronavirus spike protein and acquisition of fusion competence. Viruses. 2012;4:557–580. doi: 10.3390/v4040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirchdoerfer R.N., Cottrell C.A., Wang N., et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jardetzky T.S., Lamb R.A. Activation of paramyxovirus membrane fusion and virus entry. Curr Opin Virol. 2014;5:24–33. doi: 10.1016/j.coviro.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccines and Related Biological Products Advisory Committee Meeting. December 18, 2020. FDA briefing document-moderna COVID-19 vaccine.https://www.fda.gov/media/144434/download [Google Scholar]
- 16.Tai D.B.G., Shah A., Doubeni C.A., Sia I.G., Wieland M.L. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa815. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mulligan M.J., Lyke K.E., Kitchin N., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 18.Vaccines and Related Biological Products Advisory Committee Meeting. December 11,2020. FDA briefing document-pfizer-BioNTech COVID-19 vaccine.https://www.fda.gov/media/144245/download [Google Scholar]
- 19.Garland S.M., Hernandez-Avila M., Wheeler C.M., et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T., Matson D.O., Dennehy P., et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 21.Dexter P.R., Perkins S.M., Maharry K.S., Jones K., McDonald C.J. Inpatient computer-based standing orders vs physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA. 2004;292:2366–2371. doi: 10.1001/jama.292.19.2366. [DOI] [PubMed] [Google Scholar]
- 22.Rao S., Fischman V., Kaplan D.W., Wilson K.M., Hyman D. Evaluating interventions to increase influenza vaccination rates among pediatric inpatients. Pediatr Qual Saf. 2018;3:e102. doi: 10.1097/pq9.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States 2020. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fpfizer%2Fclinical-considerations.html#pregnant [updated December 30, 2020; cited 2021 January 4]. Available from:
- 24.Boehmer T.K., DeVies J., Caruso E., et al. Changing age distribution of the COVID-19 pandemic—United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1404–1409. doi: 10.15585/mmwr.mm6939e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein-Zamir C., Abramson N., Shoob H., et al. A large COVID-19 outbreak in a high school 10 days after schools' reopening, Israel, May 2020. Euro Surveill. 2020;25:2001352. doi: 10.2807/1560-7917.ES.2020.25.29.2001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel A., Feltman D.M., Paquette E.T. Integrating public health ethics into shared decision making for children during the novel coronavirus disease-19 pandemic. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.11.061. online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins R. The New York Times; December 30, 2020. U.S. Officials say covid-19 vaccination effort has lagged. [Google Scholar]
- 28.Centers for Disease Control and Prevention Births and natality-2018. 2018. https://www.cdc.gov/nchs/fastats/births.htm Available from:
- 29.Centers for Disease Control and Prevention Flu vaccination coverage, United States, 2019–20 influenza season atlanta, GA2020. https://www.cdc.gov/flu/fluvaxview/coverage-1920estimates.htm Available from:
- 30.Kwok K.O., Lai F., Wei W.I., Wong S.Y.S., Tang J.W.T. Herd immunity—estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020;80:e32–e33. doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kritz F. Trusted messengers, trusted messages’: how to overcome vaccine hesitancy. Natl Public Radio. December 24, 2020 https://www.npr.org/sections/health-shots/2020/12/24/948776228/trusted-messengers-trusted-messages-how-to-overcome-vaccine-hesitancy [Google Scholar]
- 32.Doucleff M. Goats and Soda; 2020. Poor Countries Fall behind in Race to Reserve COVID-19 Vaccine.https://www.npr.org/sections/goatsandsoda/2020/11/05/931397094/poor-countries-fall-behind-in-race-to-reserve-covid-19-vaccine [Internet].Available from: [Google Scholar]
- 33.World Health Organization COVAX: working for global equitable access to COVID-19 vaccines 2020. https://www.who.int/initiatives/act-accelerator/covax Available from:
- 34.Cohen J., Kupferschmidt K. As COVID-19 vaccines emerge, a global waiting game begins. Science. 2020;370:1385–1387. doi: 10.1126/science.370.6523.1385. [DOI] [PubMed] [Google Scholar]
- 35.Voysey M., Clemens S.A.C., Madhi S.A., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]