Abstract
Pernio or chilblains is characterized by erythema and swelling at acral sites (eg, toes and fingers), typically triggered by cold exposure. Clinical and histopathologic features of pernio are well described, but the pathogenesis is not entirely understood; vasospasm and a type I interferon (IFN-I) immune response are likely involved. During the coronavirus disease 2019 (COVID-19) pandemic, dermatologists have observed an increase in pernio-like acral eruptions. Direct causality of pernio due to COVID-19 has not been established in many cases because of inconsistent testing methods (often negative results) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, a form of COVID-19‒associated pernio (also called COVID toes) is probable because of increased occurrence, frequently in young patients with no cold exposure or a history of pernio, and reports of skin biopsies with positive SARS-CoV-2 immunohistochemistry. PubMed was searched between January 1, 2020, and December 31, 2020 for publications using the following keywords: pernio, chilblain, and acral COVID-19. On the basis of our review of the published literature, we speculate that several unifying cutaneous and systemic mechanisms may explain COVID-19‒associated pernio: (1) SARS-CoV-2 cell infection occurs through the cellular receptor angiotensin-converting enzyme 2 mediated by transmembrane protease serine 2, subsequently affecting the renin-angiotensin-aldosterone system with an increase in the vasoconstricting, pro-inflammatory, and prothrombotic angiotensin II pathway. (2) Severe acute respiratory syndrome coronavirus 2 cell infection triggers an immune response with robust IFN-I release in patients predisposed to COVID-19‒associated pernio. (3) Age and sex discrepancies correlated with COVID-19 severity and manifestations, including pernio as a sign of mild disease, are likely explained by age-related immune and vascular differences influenced by sex hormones and genetics, which affect susceptibility to viral cellular infection, the renin-angiotensin-aldosterone system balance, and the IFN-I response.
Abbreviations and Acronyms: ACE2, angiotensin-converting enzyme 2; ADAM17, a disintegrin and metalloproteinase 17; ANG, angiotensin; ANG1-7, angiotensin-(1-7); ANGII, angiotensin II; AT1R, angiotensin type 1 receptor; AT2R, angiotensin type 2 receptor; COVID-19, coronavirus disease 2019; HIF-1α, hypoxia-inducible factor 1α; IFN, interferon; IFN-I, type I interferon; IFN-α, interferon α; IL, interleukin; MxA, myxovirus resistance protein A; NO, nitric oxide; nsp, nonstructural protein; PCR, polymerase chain reaction; pDC, plasmacytoid dendritic cell; RAAS, renin-angiotensin-aldosterone system; S1, spike protein 1; S2, spike protein 2; SARS-CoV, severe acute respiratory syndrome coronavirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TH17, helper T cell 17; TLR7, toll-like receptor 7; TMPRSS2, transmembrane protease serine 2
Article Highlights.
-
•
One of the most common cutaneous manifestations associated with coronavirus disease 2019 (COVID-19) is pernio or chilblains, which has previously been associated with vasospasm and a type I interferon response.
-
•
Angiotensin-converting enzyme 2 (ACE2) is the cellular receptor for severe acute respiratory syndrome coronavirus 2, which is processed differently by the proteases transmembrane protease serine 2 (ACE2 cleavage facilitates viral cellular entry) and a disintegrin and metalloproteinase 17 (cleaves cell-bound ACE2, releasing an active form into the circulation). Transmembrane protease serine 2 is stimulated by androgens, whereas a disintegrin and metalloproteinase 17 is stimulated by estrogens; expression of both proteases increases with aging and inflammation.
-
•
Age and sex affect the response to COVID-19 infection because of differences in sex hormone activity, endothelial function, and innate immunity. Adult male patients and the aged exhibit more pathogenic activity of transmembrane protease serine 2, angiotensin II, and interleukin 6; female patients and the young exhibit more protective activity of angiotensin-(1-7) and type I interferon.
-
•
The complete renin-angiotensin-aldosterone system resides in the skin and includes angiotensin II, which is involved in the cutaneous thermoregulatory vasoconstriction response, and ACE2, expressed in cutaneous endothelial cells and eccrine epithelial cells, both of which may be involved in the pathogenesis of COVID-19‒associated pernio.
-
•
Through an understanding of the interconnected cutaneous and systemic mechanisms, the varying skin manifestations of COVID-19 provide important signs of disease severity and may assist in unifying the therapeutic algorithm.
Pernio or chilblains is a well-described cold-induced dermatosis, characterized by erythema and swelling localized to acral areas, occurring most commonly on the toes and fingers (Figures 1 and 2 ).1 Etymologic origins for these terms are from Latin (perna [leg]), Greek (-osis [abnormal condition]), and English (chil [cold] and blain [skin swelling]).2 Proposed diagnostic criteria include required major criteria (localized erythema and swelling involving acral sites and persistence for >24 hours) and at least 1 of the following minor criteria: onset or worsening in cooler months, consistent histopathology, and response to conservative warming treatments.3 Various laboratory abnormalities, including cold agglutinins and antiphospholipid antibodies, may accompany pernio, but their clinical importance is often unclear; occasionally, associated rheumatologic and hematologic conditions occur.3
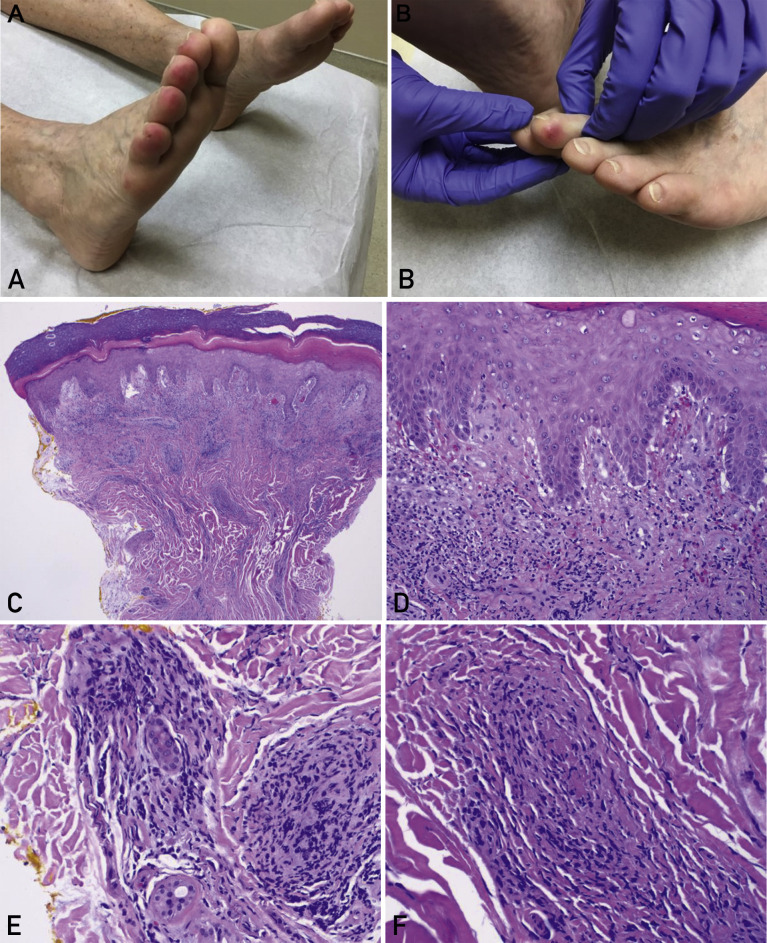
Figure 1.
Pernio in a female patient. A woman, who was in her 70s, was evaluated in Florida in February 2020. She had a history of pernio related to rheumatoid arthritis, with chronic waxing and waning tender lesions on her toes, exacerbated by wearing sandals in an air-conditioned indoor environment. Coincidentally, during the coronavirus disease 2019 (COVID-19) pandemic, she later received an unrelated diagnosis of COVID-19. She did not require hospitalization and recovered as an outpatient. Interestingly, she reported no clinically significant worsening of pernio during this viral respiratory illness, possibly because her rheumatoid arthritis was treated with tofacitinib, a Janus kinase inhibitor, which may have inhibited the effect of signal transducer and activator of transcription 1‒dependent type I interferons thought to play a role in the pathophysiology of pernio and COVID-19. A and B, Clinical photographs of the right foot (panel A) and left foot (panel B) illustrate erythematous edematous plaques affecting the distal toes. Courtesy of Ines Kevric O’Shaughnessy, MD, First Coast Dermatology Associates, Jacksonville Beach, FL; used with permission. C-F, Histopathologic sections of the patient’s punch biopsy specimen (hematoxylin-eosin) exhibit a superficial and deep dermal lymphocytic inflammatory infiltrate (panel C; original magnification, ×40); lichenoid interface dermatitis along the dermal-epidermal junction with basal vacuolar changes (panel D; original magnification, ×200); perivascular and perieccrine inflammation (panel E; original magnification, ×200); and focal lymphocytic vasculitis with fibrin thrombi involving a small dermal vessel (panel F; original magnification, ×400).
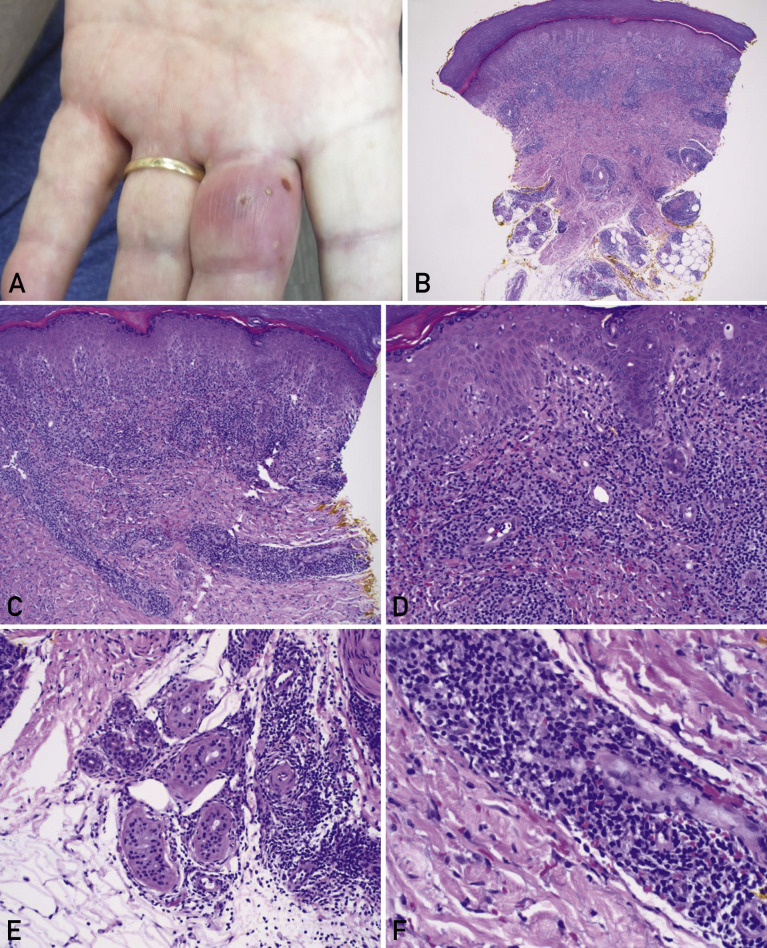
Figure 2.
Pernio in a male patient. A man who was in his 70s was evaluated in Florida in February 2020. He reported a few intermittent flares of an inflamed lesion, which affected only the long finger of the left hand. He had no history of pernio, cold exposure, autoimmune disease, travel history, or testing for coronavirus disease 2019 (COVID-19). He presented the week before the first positive case of COVID-19 was confirmed in Florida,1 so an association with COVID-19 is unlikely, unless unrecognized community spread had occurred. A, Clinical photograph of the left hand illustrates an erythematous edematous plaque with focal vesiculation affecting the long finger of the left hand. Courtesy of James B. Connors, MD, BayCare Medical Group, Sun Coast Medical Clinic Dermatology, Saint Petersburg, FL; used with permission. B-F, Histopathologic sections of the patient’s punch biopsy specimen (hematoxylin-eosin) illustrate a superficial and deep dermal lymphocytic inflammatory infiltrate (panel B; original magnification, ×40); brisk perivascular inflammation in the superficial to mid dermis (panel C; original magnification, ×100); lichenoid interface dermatitis along the dermal-epidermal junction with basal vacuolar changes (panel D; original magnification, ×200); perieccrine lymphocytic inflammation at the junction of the deep reticular dermis and the subcutaneous adipose tissue (panel E; original magnification, ×200); and focal lymphocytic vasculitis involving a small dermal vessel, with endothelial swelling and extravasation of red blood cells into the surrounding dermis (panel F; original magnification, ×400).
A related but distinct condition is chilblain lupus, a subtype of chronic cutaneous lupus erythematosus in acral locations, which is also induced by cold exposure; however, lupus-specific findings may be found on routine skin histopathology or direct immunofluorescence.4 Chilblain lupus should not be confused with lupus pernio, which is sarcoidosis that clinically resembles pernio when it occurs on the acral surfaces of the nose, cheeks, and ears.5 All pernio-like eruptions do not necessarily equate to a diagnosis of pernio, because pernio may broadly refer to acral lesions, which have many causes. Diagnostic criteria, including histopathology, are therefore essential for meaningful definitions and discussions of pernio or chilblains.
Dermatologic Manifestations of Coronavirus Disease 2019
The highly contagious and deadly coronavirus disease 2019 (COVID-19), due to severe acute respiratory syndrome coronavirus (SARS-CoV) 2 (SARS-CoV-2), has profoundly affected all medical specialties, including dermatology, necessitating new perspectives on patient and provider safety. As with many other respiratory viruses, patients with COVID-19 may develop viral exanthemata and other cutaneous manifestations. Initially, limitations on in-person dermatology evaluations and the increased complexities of performing ancillary testing, including skin biopsies, hampered understanding the pathophysiology of these pandemic-associated dermatoses.
In spite of these challenges, dermatologists rapidly shared their clinical experience and observed an increase in pernio or chilblain-like acral eruptions uncharacteristic of the spring season.6 , 7 For example, in a nationwide study from Spain, COVID-19‒associated cutaneous eruptions were clinically categorized, and pseudo-chilblains (acral erythema-edema) was the second most common finding, after maculopapular. Other dermatologic presentations (in order of reported frequency) included urticarial, vesicular, and livedoid or necrotic skin lesions.6 Similarly, in a nationwide study from France, acral lesions (chilblains or dyshidrosis-like) were the most common, with other COVID-19‒associated skin manifestations categorized as (in order of reported frequency) vesicular, urticarial, morbilliform, petechial, and livedo reticularis.7 Terms used to describe COVID-19‒associated acral eruptions include acro-ischemia, erythema multiforme‒like, dyshidrosis-like, pseudo-chilblains, and chilblain-like.6, 7, 8 The term COVID toes is popular, particularly in the mass media.9
To further understand this phenomenon, PubMed was searched for cases published in between January 1, 2020, and December 31, 2020 by using the following keywords: pernio, chilblain, and acral COVID-19. The publications were reviewed for patient characteristics, SARS-CoV-2 testing results, skin involvement (sites and biopsy results), laboratory testing, and severity of COVID-19 symptoms. Publications reporting possible COVID-19–associated pernio cases and testing results were included in the Supplemental Table (available online at http://www.mayoclinicproceedings.org).
Histopathology of Pernio
One reason for the nonuniform use of terminology for COVID-19–associated acral eruptions was the initial lack of understanding of the microscopic inflammatory pattern.8 However, the first report of the histopathologic findings10 and subsequent articles have confirmed the typical skin biopsy findings of pernio. These include a superficial and deep lymphocytic inflammatory infiltrate in a lichenoid, perivascular, and perieccrine distribution.10, 11, 12 The acral presentation of pernio frequently raises concern for primary vasculitis or thrombotic vasculopathy, and cases have been labeled as such during the pandemic13; however, the presence of a prominent perivascular lymphocytic infiltrate is consistent with pernio. Pernio may exhibit so-called lymphocytic vasculitis14 involving small dermal vessels, with endothelial swelling, fibrin thrombi, and erythrocyte extravasation15; these findings also occur in chilblain-like lesions associated with COVID-19.11 , 12 However, this is not the most common or predominant inflammatory finding in pernio regardless of association,11 , 12 , 15 and evidence is lacking to categorize pernio as systemic vasculitis.16 The term acro-ischemia is not an accurate description for the acral erythema-edema of pernio. However, severe COVID-19 is associated with acro-ischemia when presenting with livedoid to retiform purpura or necrosis at acral sites17, 18, 19 and exhibiting primary vasculopathy without the brisk lymphocytic infiltrate of pernio on skin biopsy.20
Pathophysiology of Pernio
Pernio was recognized as a diagnostic entity well before the COVID-19 pandemic, although the pathogenesis of pernio is not entirely understood. Previous clues were found in familial chilblain lupus, which is an autosomal dominant form due to sequence variations in the 3′ repair exonuclease 1 that protects cells from innate immune activation, including induction of type I interferons (IFN-Is) (eg, interferon α [IFN-α] and interferon β), which, if constitutively activated, can interfere with immune tolerance and provoke an autoimmune response.21 In the cells of patients with familial chilblain lupus, exposure to cold increased oxidative stress and activation of IFN-Is, prompting a switch to a pro-inflammatory state.21 In patients with idiopathic pernio, vasospasm occurred with ice water immersion, suggesting that vasospasm likely contributes to the pathogenesis of pernio.22 Type I interferons may inhibit the endothelial nitric oxide (NO) synthase pathway,23 a potential explanation for the vasospasm in pernio. Cryoproteins (cryoglobulins, cryofibrinogens, and cold agglutinins) have been described in childhood pernio.24 Additionally, cryofibrinogenemia has been found in 3′ repair exonuclease 1–related disease25 and chilblains during the COVID-19 pandemic,26 pointing to cryofibrinogens as an acute phase reactant because of the IFN-I response. The association of COVID-19 and chilblain-like lesions raises the question as to why SARS-CoV-2 may trigger a lymphocytic inflammatory response at acral sites; the answer may provide additional insights into the pathogenesis of pernio.
Pernio During the COVID-19 Pandemic
An international dermatology registry was created to assist in documenting the dermatologic manifestations associated with COVID-19.27 Of 505 patients with cutaneous eruptions, 318 (63%) were reported as having pernio-like eruptions, of whom 94% had on the feet, 98% received outpatient care only, 55% were asymptomatic, and 45% had respiratory COVID-19 symptoms (mostly mild).27 However, 6 patients were hospitalized, including 2 who died. Seven patients had dermatopathology, all reporting features consistent with pernio. The median age of patients was 25 years, and 29% lived where the average monthly temperature was above 10°C. Most patients (72%) had a suspected diagnosis of COVID-19, but lacked confirmatory testing owing to access limitations; only 23 (7%) had positive results for SARS-CoV-2 polymerase chain reaction (PCR) or antibody/assay testing (46 PCR negative; 14 antibody testing negative). Despite these findings, the authors concluded that the large number of reported cases of pernio during the COVID-19 pandemic was probably not merely coincidental and questioned the sensitivity of the available testing methods in patients with mild or asymptomatic disease. However, the authors conceded that even with this large case series, they could not establish causation or exclude an epiphenomenon.27 In a follow-up report of COVID-19–associated dermatologic manifestations from this international registry, 171 of 716 cases (24%) were laboratory-confirmed positive (135 by PCR; 36 by antibody/assay testing), with 31 patients (18%) having pernio-like clinical morphology.28 Compared with other cutaneous eruptions, pernio had a longer course of skin lesions but fewer and less severe COVID-19 symptoms, which is contrasted with the 11 patients with retiform purpura (6%) who all required hospitalization and respiratory support.28 In a subgroup of patients with COVID-19–associated pernio with information on the timing of SARS-CoV-2 testing, PCR positivity occurred at a median of 8 days, PCR negativity at a median of 14 days, and antibody positivity at a median of 27 days after the onset of pernio.29
Severe Acute Respiratory Syndrome Coronavirus 2 Testing in COVID-19‒Associated Pernio
It is unusual that most of the reported cases of COVID-19‒associated pernio have occurred in younger patients with no history of pernio and in warmer weather conditions than is typical of cold-induced pernio, pointing to COVID-19 as the most likely cause.30 One hypothesis is that an adequate early IFN-I response to COVID-19 occurs in younger patients,10 possibly explaining why SARS-CoV-2 PCR results are frequently negative when patients present with chilblains.30 For example, of 22 children and adolescents presenting with chilblains to an emergency department in Spain, only 1 of 19 tested had positive SARS-CoV-2 PCR results.30 Additionally, an Italian group initially reported chilblain-like lesions in 4 patients31 and subsequently reported 45 more patients with similar acral lesions32; all patients tested negative by SARS-CoV-2 PCR, and only 1 of 8 tested had IgG antibodies to SARS-CoV-2 spike protein 1 (S1) and spike protein 2 (S2).32
A Spanish group questioned the association of pernio with COVID-19 because 38 of 39 tested patients presenting with acral skin lesions had negative SARS-CoV-2 PCR results,33 and an Italian group concluded that 8 pediatric patients had primary chilblains related to cold exposure during the lockdown because none had viral respiratory symptoms, known COVID-19 contacts, or detectable SARS-CoV-2 by PCR or antibody testing.34 In another Italian series of 19 patients with histologically confirmed chilblains, 6 patients had IgA antibodies and 1 patient had IgG antibodies to SARS-CoV-2 S1, although IgG antibodies to the nucleocapsid protein of SARS-CoV-2 were negative in all patients.11 A French group also reported that IgA antibodies to SARS-CoV-2 were more frequent in 40 patients presenting with chilblains, which were found in 8 of 12 patients who were antibody positive, even though PCR results were negative in all 26 tested patients.35
The frequently negative SARS-CoV-2 testing results raise the question of the sensitivity of the available COVID-19 tests, particularly for patients with strong innate immunity that may not lead to a measurable humoral immune response, such as younger patients who more frequently present with pernio.36 , 37 In a population-based study in Switzerland, children (aged 5-9 years) had the lowest seroprevalence of SARS-CoV-2 (1%), even though 17% had at least 1 seropositive household member.38 Additionally, symptom severity is likely correlated with the degree of antibody response. For example, a study reported higher antibody titers to SARS-CoV-2 in the severe COVID-19 group than in the nonsevere group, with a significant difference in IgG titers 2 weeks after symptom onset (P=.001).39 In this same study, among 164 close contacts of patients with known COVID-19, virus-specific IgG and/or IgM were positive in 23 (14%) approximately 30 days after exposure (10 were asymptomatic); 16 (10%) also had positive PCR results (3 were asymptomatic).39 In another study including 37 asymptomatic patients, 30 (81%) had virus-specific IgG antibodies, although they had lower antibody levels than symptomatic patients during the acute phase of infection.40 In addition, 12 of 30 asymptomatic patients (40%) became seronegative in the early convalescent phase,40 and asymptomatic patients had lower levels of pro-inflammatory cytokines, including interleukin (IL)-6, than did symptomatic patients.40 In a report of 34 patients who had mild COVID-19 and at least 2 serial anti–SARS-CoV-2 antibody measurements, the average slope of a linear regression model indicated a rapid decline in antibody levels over approximately 90 days.41 Of 156 health care personnel with positive baseline SARS-CoV-2 antibodies, 146 had decreased antibody levels at approximately 60 days of follow-up and 44 had seroreversion, which was more common if they had a lower baseline antibody level or were asymptomatic for COVID-19.42
T cells targeting SARS-CoV-2 are an important aspect of the immune response; they occur in most convalescent patients with COVID-19 (including those with mild infection) and in a subset of unexposed individuals, likely indicating cross-reactivity to common cold coronaviruses.43, 44, 45 Both home contacts (who had negative SARS-CoV-2 antibodies) and family members with mild COVID-19 (who had positive SARS-CoV-2 antibodies) have had SARS-CoV-2‒specific interferon γ‒producing T cells, suggesting that the T-cell response is a more sensitive indicator of COVID-19 exposure than antibody seroconversion.46 The lack of antibodies may be attributed to a robust innate immune response because sustained IFN-I activity inhibits viral replication, antigen presentation, and an adaptive B-cell antibody response.47 Another factor may be preexisting cross-reactive coronavirus antibodies that can target SARS-CoV-2, which have been found in uninfected individuals and are more prevalent at younger ages.48 Cross-reactive immune protection was also suggested in hospitalized patients with COVID-19 and previously detected endemic coronavirus, because they had lower odds of intensive care unit admission and higher survival probability.49
Severe Acute Respiratory Syndrome Coronavirus 2 Testing in Skin Biopsies of COVID-19‒Associated Pernio
Coronavirus disease 2019‒associated chilblain-like lesions have exhibited perivascular and perieccrine lymphocytic infiltrates of predominantly CD3+ T cells, with collections of CD123+ or CD303+ plasmacytoid dendritic cells (pDCs).11 , 12 Plasmacytoid dendritic cells produce IFN-I and are thought to be involved in the pathogenesis of chilblain lupus and COVID-19–associated pernio.50 CD123+ pDCs51 and expression of myxovirus resistance protein A (MxA), a marker of IFN-I signaling, are found in chilblain lupus, idiopathic pernio, and COVID-19–associated pernio.20 , 52 Positive staining for phosphorylated Janus kinase, an indicator of IFN receptor activation, has also been found in the cutaneous epithelium and endothelium of chilblain-like lesions associated with the COVID-19 pandemic.53 Higher IFN-α levels after in vitro stimulation were observed in 25 patients with chilblain-like lesions during the COVID-19 pandemic who were SARS-CoV-2 PCR negative compared with ambulatory and hospitalized patients with PCR-positive COVID-19.54 Direct immunofluorescence has revealed dermal vascular deposits of C3, confirming complement activation in COVID-19–associated pernio.11 , 12
During the COVID-19 pandemic, positive immunohistochemical staining of cutaneous vascular endothelium and eccrine epithelium with a SARS-CoV/SARS-CoV-2 spike protein antibody has been exhibited in patients with pernio-like lymphocytic infiltrates on skin biopsy.55, 56, 57 However, discrepant skin immunohistochemistry for SARS-CoV-2 was reported in a case series of 5 pernio patients: 0 of 5 had positive staining with a nucleocapsid protein antibody, 0 of 3 had positive staining with RNA in situ hybridization to the spike protein, and 3 of 5 had positive staining with a spike protein antibody.58 , 59 Another series reported positive immunohistochemistry with a SARS-CoV-2 nucleocapsid protein antibody in the eccrine glands of 3 patients with chilblain-like histopathology.60 Additionally, coronavirus-like particles within the cytoplasm of endothelial cells56 and rare SARS-CoV-2 RNA‒positive cells20 have been found in skin biopsies of COVID-19–associated pernio. Polymerase chain reaction testing for SARS-CoV-2 in skin biopsies of chilblains during the COVID-19 pandemic is frequently negative,61 although one report detected SARS-CoV-2 and increased kallikrein by PCR from a chilblain-like lesion of the thumb.62
Colmenero et al56 attributed direct causality to COVID-19 in their patients with chilblains, favoring the hypothesis of widespread endothelial infection by SARS-CoV-2 leading to resultant endothelial damage and thrombosis, contending that this argues against the hypothesis that describes the role of IFN-I in the pathogenesis of COVID-19‒associated pernio. These potential mechanisms, however, are not necessarily mutually exclusive and may be interdependent; SARS-CoV-2 is not the necessary cause of pernio because this diagnosis preexisted the COVID-19 pandemic. Lipsker63 proposed that chilblains is a paraviral eruption associated with COVID-19, a concept distinguished from classic viral exanthems in being defined by clinically recognizable morphology with multiple potential etiologies, which is persistent or delayed owing to the immune reaction rather than specific viral cytopathic effect.
Role of Angiotensin-Converting Enzyme 2 in COVID-19
Angiotensin-converting enzyme 2 (ACE2) functions as the receptor on cells that mediate cellular entry for both SARS-CoV and SARS-CoV-2.64 , 65 First the viral protein subunit S1 binds to the receptor ACE2; the second step is protein cleavage of the S1 and S2 protein subunits, which is completed by the transmembrane protease serine 2 (TMPRSS2).66 , 67 After the S1 protein subunit separation, the remaining S2 protein subunit conformationally rearranges, which allows the fusion of the viral and cellular membranes and subsequent cellular entry of the virus.66 , 67 This process leads to down-regulation of ACE2 on cells because it is functionally removed from the external membrane site.67 Angiotensin-converting enzyme 2 is primarily membrane bound on cells, although it is also detectable in lower quantities as a circulating soluble form.67 A disintegrin and metalloproteinase 17 (ADAM17) also cleaves membrane-bound ACE2, releasing an active form into the circulation and leaving an inactive portion on the cell membrane.67 , 68 Transmembrane protease serine 2 competes with ADAM17 for ACE2 processing but cleaves ACE2 differently, so that only TMPRSS2 facilitates SARS-CoV cell entry.65 , 69
Cutaneous ACE2 and COVID-19‒Associated Pernio
Angiotensin-converting enzyme 2 messenger RNA expression occurs in the skin and is positively correlated with the expression of immune signature genes of lymphocytes and the IFN response.70 In addition, ACE2 protein expression in the skin has been revealed by immunohistochemistry, which exhibits strong staining of the basal layer of the epidermis and hair follicles, the dermal blood vessels, and the eccrine glands.71 In all forms of pernio, the lymphocytic infiltrate characteristically exhibits lymphocytes at the dermal-epidermal junction along the basal layer and in a perivascular and perieccrine distribution,11 , 12 , 15 curiously centered around these areas of ACE2 protein expression. Single-cell RNA sequencing of epidermal keratinocytes has exhibited the expression of ACE2 and TMPRSSs in normal human skin and SARS-CoV-2 nucleocapsid protein in patients with COVID-19.72
Renin-Angiotensin-Aldosterone System Imbalance in COVID-19
Although cell-bound ACE2 allows cellular entry for SARS-CoV-2, ACE2 also provides a vasoprotective function by converting angiotensin (ANG) II (ANGII) to ANG-(1-7) (ANG1-7).67 Increased levels of ANGII lead to endothelial dysfunction by binding ANG type 1 receptor (AT1R) and resulting in increased aldosterone release, vasoconstriction, coagulation, immune cell activation, and inflammatory cytokines.67 These effects are opposed by ANG1-7 binding the ANG type 2 receptor (AT2R) and the Mas receptor, which promotes healthy endothelial function through increased levels of NO, vasodilation, and an antithrombotic and anti-inflammatory state.67 These downstream-positive effects of ACE2 in balancing the renin-angiotensin-aldosterone system (RAAS) lead to the paradox that despite being the viral receptor, ACE2 likely has vasoprotective effects.
Discrepancies in COVID-19 Severity by Sex and Age
Severe COVID-19 occurs more frequently in male patients73 , 74 and older patients.75 Differences in the RAAS may be one explanation, as in male patients and older adults the angiotensin-converting enzyme–driven ANGII-AT1R axis is favored,76 , 77 whereas in female patients the balance is shifted toward increased activity of ACE2 and the positive effects of ANG1-7 binding AT2R and the Mas receptor.76 Estradiol increases ANG1-7 production through estrogen receptor α and increases ACE2 expression and activity.78 In addition, estrogens are vasoprotective and preserve the presence and activity of endothelial NO synthase but this NO-producing pathway becomes dysfunctional with aging.79
Sex hormone and genetic differences affecting the degree of androgen sensitization likely play a role in COVID-19 severity.80 This is because estradiol enhances the expression of ADAM17 through the estrogen receptor81 whereas TMPRSS2 is regulated by androgens, including dihydrotestosterone, through the androgen receptor,82 with TMPRSS2 facilitating SARS-CoV entry into cells69 and increasing expression in the lung epithelium with age.83 The stimulatory effects of estradiol on ADAM17 result in the cleaving and shedding of membrane-bound IL-6 receptor into the soluble form and diminish glycoprotein 130 expression, thereby inhibiting IL-6 signaling.84 , 85 In addition, pDCs from female patients, compared with male patients, have higher levels of expression of all subtypes of IFN-α and surface expression of the IFN-α/interferon β receptor subunit 2.86 In young individuals, female sex and postpuberty are associated with increased pDC activation and toll-like receptor 7 (TLR7)–induced production of IFN-α, related to X chromosome number and the differential effect of serum testosterone concentration.87
The TLR7 gene is present on the X chromosome and escapes X chromosome inactivation, resulting in biallelic expression in a proportion of female immune cells and an increased IFN-I response.88 In a cohort of patients with COVID-19, higher IFN-α2 levels were found in female patients than in male patients; female patients (but not male patients) also had higher IFN-α2 levels than did sex-matched health care worker controls.89 In contrast, in a case series of 4 young men (2 pairs of brothers younger than 35 years) with an X-linked loss-of-function TLR7 sequence variation resulting in down-regulated IFN-I signaling, all had severe COVID-19 and required ventilatory support.90 Genetic defects in various IFN-I immune pathway genes,91 including the IFN receptor92 and autoantibodies against IFN-Is found more commonly in men,93 have been found in subsets of patients with life-threatening COVID-19.
As people age, dendritic cells secrete less IFN-I94 and serum levels of IL-6 increase in both sexes; although testosterone decreases in male patients and estradiol decreases in female patients, both sex hormones may inhibit IL-6 activity.95 , 96 Therefore, in older patients, the IL-6 pathway may predominate; higher IL-6 levels are associated with more severe COVID-19.97 When the immune response to SARS-CoV-2 was compared between pediatric and adult patients, adult patients mounted a more robust T-cell and neutralizing antibody response to the viral spike protein, suggesting that an early innate immune response may be more important in younger patients with COVID-19.98 , 99 In addition to the immune differences in children, there are several other potential explanations, including endothelial function, for the age-related differences in COVID-19 severity.100
Type I Interferons and pDCs in COVID-19
Type I interferons are primarily produced by pDCs, which provide an important link between innate and adaptive immunity.101 Plasmacytoid dendritic cells are considered sentinel cells102 that are stimulated upon physical contact with virally infected cells at an adhesion site (an interferogenic synapse).103 Through this contact synapse, viral RNA transfer to pDCs leads to TLR7 signaling and production of IFN-I by pDCs, which may be locally secreted on infected cells.103 However, in chronic viral infection or autoimmune disease, pDCs are persistently activated, contributing to disease pathogenesis through excessive IFN-I activity.104 Persistent viral infection may subsequently impair pDCs and in turn lead to diminished virus-specific T-cell responses.105
Lymphopenia is a common finding in COVID-19; all lymphocyte subsets are affected, and lower lymphocyte counts are associated with more severe disease.106 Severe COVID-19 is associated with a sustained decrease in lymphocytes, and neutrophil counts and IL-6 levels are higher than those in mild cases.107 Severe acute respiratory syndrome coronavirus 2 is composed of 27 viral proteins, including nonstructural proteins (nsps), structural proteins, and accessory proteins; nsp13, nsp14, nsp15, and the open reading frame orf6 function as IFN antagonists.108 The timing and degree of the IFN-I response likely explains the disease severity of COVID-19.101 In comparison to patients with mild-to-moderate COVID-19, patients with severe-to-critical disease and a higher plasma viral load exhibit lower IFN-I–stimulated gene expression and IFN-α serum levels.109 Interestingly, IFN-Is up-regulate ACE2 in human airway epithelial cells, and SARS-CoV-2 may exploit this tissue-protective response by providing more receptor targets on cells for viral entry.110
Relation of Antiphospholipid Antibodies in COVID-19‒Associated Pernio
Lupus anticoagulant and antiphospholipid antibodies have been reported to be frequently positive in hospitalized patients with COVID-19.111, 112, 113, 114 Viral infections can trigger the development of antiphospholipid antibodies, probably through molecular mimicry, with most cases being transient and nonpathogenic; however, catastrophic antiphospholipid syndrome has been associated with some viral infections.115
Antiphospholipid antibodies have been proposed as a factor in a subset of pernio patients, some of whom have eventually met the criteria for systemic lupus erythematosus.116 However, antiphospholipid antibodies are not a consistent finding in idiopathic pernio.3 In cases of COVID-19‒associated pernio, coagulation studies occasionally find mild D-dimer elevations and positive antiphospholipid antibodies.26 Conversely, acral livedoid purpura is associated with severe COVID-19 and systemic coagulopathy characterized by high elevations in D-dimer and the need for anticoagulation therapy.117
Coagulopathy, Thrombosis, T Lymphocytes, and COVID-19‒Associated Pernio
Coronavirus disease 2019 has been associated with several coagulation defects, including elevated D-dimer levels, pulmonary thrombosis, venous thromboembolism, and disseminated intravascular coagulation.118 In a prospective cohort study, patients with acute respiratory distress syndrome due to COVID-19 had increased thrombotic complications, including pulmonary embolism, despite anticoagulation.119 Some have suggested that COVID-19 may result in distinct sepsis-induced coagulopathy owing to activation of endothelial cells, inflammatory cytokines, and complement pathways.118 Complement activation has been implicated in the pathogenesis of thrombotic vasculopathy seen in severe COVID-19 with respiratory failure.120 Three of 5 such patients had livedoid purpuric skin lesions, in which the cutaneous and pulmonary microvasculature revealed similar pauci-inflammatory thrombotic vasculopathy with complement deposition,120 which in 2 cases colocalized with the SARS-CoV-2 spike protein.120 Thrombotic retiform purpura in severe COVID-19 displays extensive endothelial complement deposition and SARS-CoV-2 protein localization, with positive IL-6 and negative MxA expression; in comparison, pernio associated with mild COVID-19 displays minimal staining for complement and IL-6 but strong MxA expression.20
This spectrum of COVID-19‒associated cutaneous endothelial dysfunction may be partly due to the effects of SARS-CoV-2 on ACE2 and the RAAS. Angiotensin II is involved in microvascular thrombosis through the thrombin coagulation pathway,121 and T lymphocytes mediate accelerated ANGII-related microvascular thrombosis.122 T cells express AT1R, and ANGII thereby stimulates T-cell activation and proliferation.123 Angiotensin II–induced microvascular thrombosis and inflammatory responses are mediated by T-cell‒dependent IL-6 signaling124 through the signal transducer and activator of transcription 3 pathway.125 Angiotensin II–AT1R activation additionally stimulates aldosterone synthesis and subsequent mineralocorticoid receptor activation, resulting in endothelial dysfunction.126 The mineralocorticoid receptor is also expressed on dendritic cells, and when stimulated with aldosterone, dendritic cells secrete IL-6 and promote helper T cell 17 (TH17) polarization of T cells.127 Dendritic cells additionally express AT1R, and ANGII thereby activates dendritic cell expression of pro-inflammatory cytokines and T-cell proliferation associated with the increased phosphorylation of signal transducer and activator of transcription 1.128 Overall, the contribution of ANGII to microvascular thrombosis and T-cell activation may provide an explanation for the clinicopathologic spectrum of pauci-inflammatory thrombotic vasculopathy in severe COVID-19–associated retiform purpura and the lymphocyte-rich perivascular infiltrate in mild COVID-19‒associated pernio.
Hypoxia as a Factor in COVID-19‒Associated Pernio
Hypoxia in COVID-19 is not surprising given patients’ related pneumonia with ground glass opacities on radiologic imaging studies, although some have hypothesized that hemoglobin dysfunction may also be involved.129 Additionally, relative hypoxia may occur within other tissues, in part because of the vasoconstricting and prothrombotic effects of unopposed ANGII. The subsequent endothelial dysfunction could then, for example, result in local hypoxia of the skin and be an additional contributing factor to the pathogenesis of COVID-19–associated acral eruptions. Hypoxia-inducible factor 1α (HIF-1α) is a transcription factor that facilitates the switch in metabolic pathway in response to hypoxia and functions as a sensor of oxygen tension in inflammatory environments, which are relatively hypoxic.130 Hypoxia-inducible factor 1α promotes TH17 differentiation by increasing IL-17 in a signal transducer and activator of transcription 3–dependent fashion.130 Hypoxia-inducible factor 1α also inhibits regulatory T-cell activity by inducing forkhead box P3 protein degradation.130 Normally regulatory T cells provide an anti-inflammatory check and inhibit the development of autoimmune responses, but this activity may be overcome by tissue hypoxia, which induces a pro-inflammatory TH17 state in an HIF-1α‒dependent manner.130 Because pernio is associated with vasospasm and retiform purpura is associated with thrombosis, both of which may result in relative cutaneous hypoxia, HIF-1α could be a cofactor in the inflammatory response in COVID-19131 and associated cutaneous endothelial dysfunction.
Cutaneous Endothelial Function and RAAS in COVID-19‒Associated Pernio
Coronavirus disease 2019‒associated pernio may more commonly affect younger patients because of age-related differences in cutaneous endothelial function. The complete RAAS resides in human skin and includes ANGII, which can be synthesized locally, and its receptors AT1R and AT2R, which are found in epidermal keratinocytes and dermal vessels.132 Cutaneous vascular responses to ANGII are age-related.77 , 133 In young adults, reflex cutaneous vasoconstriction to cold exposure is primarily dependent on sympathetic nerve activity.134 However, thermoregulatory reflex cutaneous vasoconstriction attenuates with older age because of impaired skin sympathetic nerve activity.135 The result in older adults is increased reliance on ANGII-mediated vasoconstriction through AT1R stimulation of the compromised sympathetic pathways.77 The age-related differences in ANGII response also suggest that as adults age, AT1R density increases whereas AT2R density decreases, and the dose-response curve shifts in older adults, with less AT2R-mediated vasodilation (which is protective) at lower ANGII concentrations and more AT1R-mediated vasoconstriction (which is pathogenic) with increasing ANGII concentrations.77 Nevertheless, younger adults do have AT1R-mediated vasoconstriction at higher levels of ANGII, but, unlike older individuals, younger adults maintain adequate reflex cutaneous vasoconstriction to cold exposure.77 , 133 Therefore, locally increased ANGII due to ACE2 deficiency from SARS-CoV-2 infection, in combination with an intact thermoregulatory vasoconstriction response, may contribute to the acral vasopasm of pernio more commonly affecting younger patients with COVID-19.
Proposed Mechanism for COVID-19‒Associated Pernio (COVID Toes)
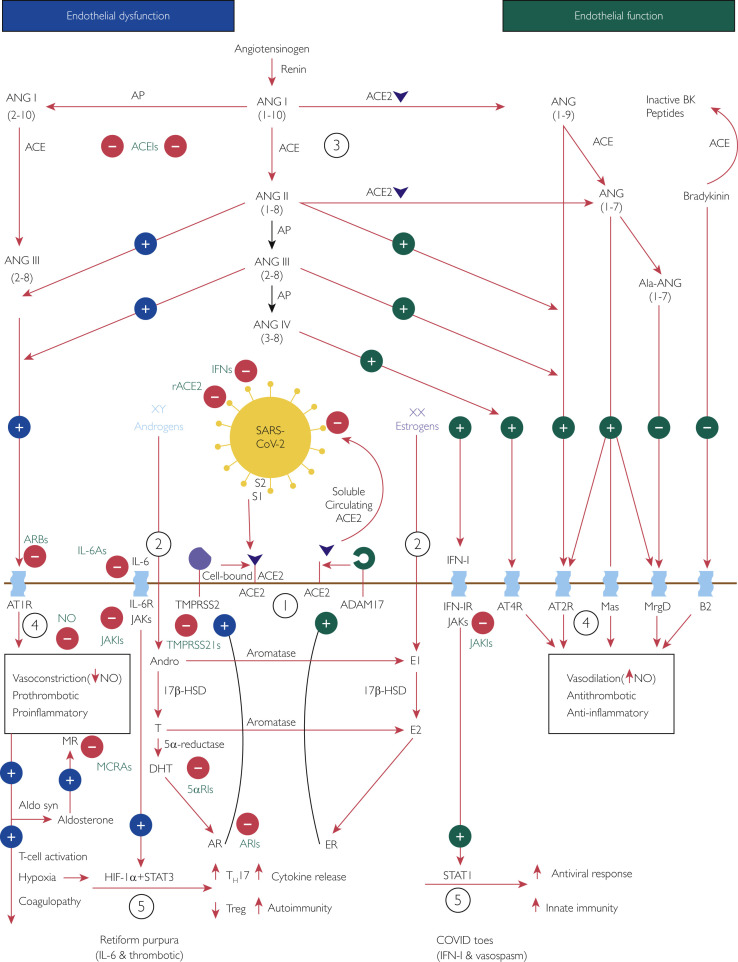
On the basis of a review of the published literature, we speculate that the mechanism for COVID-19‒associated pernio (COVID toes) involves an interplay of SARS-CoV-2 cell infection through ACE2, the RAAS, sex hormones, and the IFN-I immune response (Figure 3 64, 65, 66, 67, 68, 69 , 76 , 81 , 82 , 86 , 87 , 94 , 97 , 123, 124, 125, 126 , 128 , 130 , 136). These interconnected mechanisms provide a rationale for some of the therapeutics studied in the context of COVID-19 infection, including RAAS inhibitors, recombinant ACE2, NO-mediated vasodilators, antiandrogens, antithrombotics, anti-inflammatory agents, Janus kinase inhibitors, IL-6 antagonists, and antiviral IFNs.
Figure 3.
Interplay of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), angiotensin-converting enzyme (ACE) 2 (ACE2), the renin-angiotensin-aldosterone system (RAAS), sex hormones, and the immune response: a potential mechanism of coronavirus disease (COVID) toes. Used with permission of M.A. Cappel, MD. Circled numbers indicate steps in the mechanism. Step 1: The cellular receptor ACE2 is critically important in SARS-CoV-2 infection.64,65 In addition, transmembrane protease serine 2 (TMPRSS2) is essential because by cleaving cell-bound ACE2 and SARS-CoV-2 spike protein subunit 1 (S1) from spike protein subunit 2 (S2), it facilitates viral cellular entry.65,66,69Step 2: Androgens and estrogens have generally opposing downstream effects on ACE2 processing, providing an explanation for more severe coronavirus disease 2019 (COVID-19) in male patients. TMPRSS2 activity increases with androgen sensitization through dihydrotestosterone activation of the androgen receptor (AR).82 On the contrary, estrogens increase the expression of a disintegrin and metalloproteinase 17 (ADAM17),81 which competes for processing of ACE2 and releases a circulating form of active ACE2.68,69 Therefore, increased ADAM17 activity may be protective in female patients, resulting in an increased proportion of circulating ACE2 that binds any circulating SARS-CoV-2 and prevents further cell infection. Step 3: When cells are infected by SARS-CoV-2, the resulting virus-receptor internalization results in the decreased cell expression of ACE2 and a relative deficiency of ACE2.67 In the RAAS, a delicate balance exists between ACE and ACE2. ACE converts angiotensin (ANG) I (ANGI) to ANGII and, with aminopeptidase, to ANGIII, both of which contribute to endothelial dysfunction through binding the angiotensin type 1 receptor (AT1R).136 On the contrary, ACE2 converts ANGI to ANG1-9 and ANGII to ANG1-7, both of which promote healthy endothelial function through binding the AT2R.136 At baseline, because of sex hormone differences, the ACE–ANGII–AT1R pathway is favored in male patients whereas the ACE2‒ANG1-7‒AT2R pathway is favored in female patients.76Step 4: These RAAS predilections may account for increased endothelial dysfunction in male patients compared with female patients; AT1R stimulation decreases nitric oxide (NO) and is vasoconstricting, prothrombotic, and pro-inflammatory; AT2R stimulation increases NO and is vasodilatory, antithrombotic, and anti-inflammatory.136 Angiotensin II–AT1R activation also potentiates endothelial dysfunction by stimulating aldosterone synthesis and subsequent mineralocorticoid receptor activation, with similar vasculopathic and pro-inflammatory effects.126Step 5: Aldosterone stimulates mineralocorticoid receptors on dendritic cells, and ANGII stimulates AT1Rs on T cells and on dendritic cells; all these actions promote T-cell activation and proliferation.123 This leads to activation of interleukin 6 family (IL-6) cytokine receptors (IL-6Rs), which signal through the Janus kinase (JAK)‒signal transducer and activator of transcription (STAT) 3 pathway,124 and activation of the type I interferon (IFN-I) receptor (IFN-IR), which signals through the JAK-STAT1 pathway.125,128 Tissue hypoxia from the related AT1R-induced endothelial dysfunction may also contribute through hypoxia-inducible factor 1α subunit (HIF-1α) in conjunction with STAT3 to increase pro-inflammatory helper T cell 17s (TH17s) and decrease anti-inflammatory regulatory T cells (Tregs).130 Owing to sex hormone and genetic differences, female patients have a more robust IFN-I‒STAT1 response than do male patients.86,87 Younger individuals have a stronger IFN-I response,94 which favors the development of COVID toes; in older individuals the IL-6 pathway may predominate, which is associated with more severe COVID-19.97 5αRI, 5α-reductase inhibitor; 17β-HSD, 17β-hydroxysteroid dehydrogenase; ACEI, angiotensin-converting enzyme inhibitor; Ala, alamandine; Aldo syn, aldosterone synthase; Andro, androstenedione; AP, aminopeptidase; ARB, angiotensin receptor blocker; ARI, androgen receptor inhibitor; B2, bradykinin receptor B2; BK, bradykinin; DHT, dihydrotestosterone; E1, estrone; E2, estradiol; ER, estrogen receptor; IFN, interferon; IL-6A, IL-6 antagonist; JAKI, Janus kinase inhibitor; Mas, G protein‒coupled receptor Mas receptor; MCRA, mineralocorticoid receptor antagonist; MR, mineralocorticoid receptor; MrgD, Mas-related G protein‒coupled receptor member D; rACE2, recombinant angiotensin-converting enzyme 2; T, testosterone; TMPRSS2I, transmembrane protease serine 2 inhibitor; XX, 2 X chromosomes (genetic female); XY, 1 X chromosome and 1 Y chromosome (genetic male).
Type I interferons are antiviral by preventing viral replication, and they up-regulate ACE2 epithelial expression,110 thereby preventing ACE2 deficiency. Therefore, with robust IFN-I release in mild COVID-19, any ACE2 deficiency and subsequent increase in ANGII activity is limited, possibly triggering temporary acral vasospasm and manifesting as pernio. However, with a lessened IFN-I response, ACE2 deficiency/ANGII increase may be more pronounced, including the related endothelial dysfunction, potentially resulting in fluctuating arteriolar vasospasm (presenting as livedo reticularis137) in moderate COVID-19 or protracted vasospasm and thrombosis (presenting as livedo racemosa, retiform purpura, or acral necrosis in acro-ischemia17, 18, 19 , 120) in severe COVID-19. The greater the degree of innate IFN-I response, the more likely that SARS-CoV-2 PCR and antibody testing will be negative; therefore, skin biopsy immunohistochemistry and blood testing for viral signature markers, including IFN-I‒inducible MxA,138 and lymphocyte assays for SARS-CoV-2–reactive T cells139 deserve further investigation in patients with suspected COVID-19–associated pernio.
Limitations
The caveats of this review include the retrospective nature of most published studies; the lack of prospective data thus far is due to the novel nature of the current SARS-CoV-2 pandemic. For example, SARS-CoV-2 testing methods and results have been highly variable, as the best practices for proving infection in patients with possible cutaneous manifestations have yet to be determined. We also acknowledge that from a review of the published literature, we have made speculative hypotheses about the potential cutaneous and systemic mechanisms involved in the pathophysiology of COVID-19–associated pernio that will need to be confirmed in future prospective studies.
Conclusion
Pernio or chilblains is the most common diagnosis to explain COVID toes, because affected patients present with erythema and swelling involving acral surfaces and consistent lymphocyte-rich histopathology, fulfilling the previously proposed diagnostic criteria.3 However, it is critical to distinguish pernio from other cutaneous acral eruptions that can also be associated with COVID-19, particularly pauci-inflammatory thrombo-occlusive vasculopathy, which presents with livedoid to retiform purpura or necrotic to ulcerated acral skin lesions. Therefore, it is essential to recognize any dermatologic findings potentially associated with SARS-CoV-2 infection, which are important cutaneous signs of COVID-19 severity.
Acknowledgments
Editing, proofreading, reference verification, and illustration formatting assistance was provided by Scientific Publications, Mayo Clinic.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at: http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Florida COVID-19 response Florida Health website. https://floridahealthcovid19.gov/#latest-stats
- 2.Pernio Wiktionary website. https://en.wiktionary.org/wiki/pernio
- 3.Cappel J.A., Wetter D.A. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89(2):207–215. doi: 10.1016/j.mayocp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Su W.P., Perniciaro C., Rogers R.S., III, White J.W., Jr. Chilblain lupus erythematosus (lupus pernio): clinical review of the Mayo Clinic experience and proposal of diagnostic criteria. Cutis. 1994;54(6):395–399. [PubMed] [Google Scholar]
- 5.Neville E., Mills R.G., Jash D.K., Mackinnon D.M., Carstairs L.S., James D.G. Sarcoidosis of the upper respiratory tract and its association with lupus pernio. Thorax. 1976;31(6):660–664. doi: 10.1136/thx.31.6.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galván Casas C., Català A., Carretero Hernández G. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183(1):71–77. doi: 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Masson A., Bouaziz J.D., Sulimovic L. SNDV (French National Union of Dermatologists-Venereologists). Chilblains is a common cutaneous finding during the COVID-19 pandemic: a retrospective nationwide study from France. J Am Acad Dermatol. 2020;83(2):667–670. doi: 10.1016/j.jaad.2020.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Nieto D., Jimenez-Cauhe J., Suarez-Valle A. Characterization of acute acral skin lesions in nonhospitalized patients: a case series of 132 patients during the COVID-19 outbreak. J Am Acad Dermatol. 2020;83(1):e61–e63. doi: 10.1016/j.jaad.2020.04.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKay B., Hernandez D. Coronavirus hijacks the body from head to toe, perplexing doctors. The Wall Street Journal. May 7, 2020 [Google Scholar]
- 10.Kolivras A., Dehavay F., Delplace D. Coronavirus (COVID-19) infection-induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489–492. doi: 10.1016/j.jdcr.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Hachem M., Diociaiuti A., Concato C. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):2620–2629. doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanitakis J., Lesort C., Danset M., Jullien D. Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020;83(3):870–875. doi: 10.1016/j.jaad.2020.05.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Gil M.F., García García M., Monte Serrano J., Prieto-Torres L., Ara-Martín M. Acral purpuric lesions (erythema multiforme type) associated with thrombotic vasculopathy in a child during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020;34(9):e443–e445. doi: 10.1111/jdv.16644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herman E.W., Kezis J.S., Silvers D.N. A distinctive variant of pernio: clinical and histopathologic study of nine cases. Arch Dermatol. 1981;117(1):26–28. [PubMed] [Google Scholar]
- 15.Boada A., Bielsa I., Fernandez-Figueras M.T., Ferrandiz C. Perniosis: clinical and histopathological analysis. Am J Dermatopathol. 2010;32(1):19–23. doi: 10.1097/DAD.0b013e3181af1d24. [DOI] [PubMed] [Google Scholar]
- 16.Sunderkötter C.H., Zelger B., Chen K.R. Nomenclature of cutaneous vasculitis: dermatologic addendum to the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheumatol. 2018;70(2):171–184. doi: 10.1002/art.40375. [DOI] [PubMed] [Google Scholar]
- 17.Bosch-Amate X., Giavedoni P., Podlipnik S. Retiform purpura as a dermatological sign of coronavirus disease 2019 (COVID-19) coagulopathy. J Eur Acad Dermatol Venereol. 2020;34(10):e548–e549. doi: 10.1111/jdv.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvão J., Relvas M., Pinho A., Brinca A., Cardoso J.C. Acro-ischaemia and COVID-19 infection: clinical and histopathological features. J Eur Acad Dermatol Venereol. 2020;34(11):e653–e754. doi: 10.1111/jdv.16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Giudice P., Boudoumi D., Le Guen B. Catastrophic acute bilateral lower limbs necrosis associated with COVID-19 as a likely consequence of both vasculitis and coagulopathy. J Eur Acad Dermatol Venereol. 2020;34(11):e679–e680. doi: 10.1111/jdv.16763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro C.M., Mulvey J.J., Laurence J. The differing pathophysiologies that underlie COVID-19 associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2020;184(1):141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmermann N., Wolf C., Schwenke R. Assessment of clinical response to janus kinase inhibition in patients with familial chilblain lupus and TREX1 mutation. JAMA Dermatol. 2019;155(3):342–346. doi: 10.1001/jamadermatol.2018.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahi V., Wetter D.A., Cappel J.A., Davis M.D., Spittell P.C. Vasospasm is a consistent finding in pernio (chilblains) and a possible clue to pathogenesis. Dermatology. 2015;231(3):274–279. doi: 10.1159/000437224. [DOI] [PubMed] [Google Scholar]
- 23.Jones Buie J.N., Oates J.C. Role of interferon alpha in endothelial dysfunction: insights into endothelial nitric oxide synthase-related mechanisms. Am J Med Sci. 2014;348(2):168–175. doi: 10.1097/MAJ.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weston W.L., Morelli J.G. Childhood pernio and cryoproteins. Pediatr Dermatol. 2000;17(2):97–99. doi: 10.1046/j.1525-1470.2000.01722.x. [DOI] [PubMed] [Google Scholar]
- 25.Paradis C., Cadieux-Dion M., Meloche C. TREX-1-related disease associated with the presence of cryofibrinogenemia. J Clin Immunol. 2019;39(1):118–125. doi: 10.1007/s10875-018-0584-x. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Fernández C., López-Sundh A.E., González-Vela C. High prevalence of cryofibrinogenemia in patients with chilblains during the COVID-19 outbreak. Int J Dermatol. 2020;59(12):1475–1484. doi: 10.1111/ijd.15234. [DOI] [PubMed] [Google Scholar]
- 27.Freeman E.E., McMahon D.E., Lipoff J.B. American Academy of Dermatology Ad Hoc Task Force on COVID-19. Pernio-like skin lesions associated with COVID-19: a case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020;83(2):486–492. doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman E.E., McMahon D.E., Lipoff J.B. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman E.E., McMahon D.E., Hruza G.J. Timing of PCR and antibody testing in patients with COVID-19-associated dermatologic manifestations. J Am Acad Dermatol. 2021;84(2):505–507. doi: 10.1016/j.jaad.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andina D., Noguera-Morel L., Bascuas-Arribas M. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37(3):406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colonna C., Monzani N.A., Rocchi A., Gianotti R., Boggio F., Gelmetti C. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr Dermatol. 2020;37(3):437–440. doi: 10.1111/pde.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colonna C., Spinelli F., Monzani N.A., Ceriotti F., Gelmetti C. Chilblains in children in the time of COVID-19: new evidence with serology assay. Pediatr Dermatol. 2020;37(5):1000–1001. doi: 10.1111/pde.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Docampo-Simón A., Sánchez-Pujol M.J., Juan-Carpena G. Are chilblain-like acral skin lesions really indicative of COVID-19? A prospective study and literature review. J Eur Acad Dermatol Venereol. 2020;34(9):e445–e447. doi: 10.1111/jdv.16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neri I., Virdi A., Corsini I. Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34(11):2630–2635. doi: 10.1111/jdv.16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubiche T., Le Duff F., Chiaverini C., Giordanengo V., Passeron T. Negative SARS-CoV-2 PCR in patients with chilblain-like lesions. https://doi.org/10.1016/S1473-3099(20)30518-1 [published online ahead of print June 18, 2020]. Lancet Infect Dis. [DOI] [PMC free article] [PubMed]
- 36.Lipsker D. A chilblain epidemic during the COVID-19 pandemic: a sign of natural resistance to SARS-CoV-2? Med Hypotheses. 2020;144:109959. doi: 10.1016/j.mehy.2020.109959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahieu R., Tillard L., Le Guillou-Guillemette H. No antibody response in acral cutaneous manifestations associated with COVID-19? J Eur Acad Dermatol Venereol. 2020;34(10):e546–e548. doi: 10.1111/jdv.16688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stringhini S., Wisniak A., Piumatti G. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long Q.X., Liu B.Z., Deng H.J. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 40.Long Q.X., Tang X.J., Shi Q.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 41.Ibarrondo F.J., Fulcher J.A., Goodman-Meza D. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383(11):1085–1087. doi: 10.1056/NEJMc2025179. [published correction appears in N Engl J Med. 2020;383(11):e74] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Self W.H., Tenforde M.W., Stubblefield W.B., CDC COVID-19 Response Team; IVY Network Decline in SARS-CoV-2 antibodies after mild infection among frontline health care personnel in a multistate hospital network—12 states, April-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(47):1762–1766. doi: 10.15585/mmwr.mm6947a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grifoni A., Weiskopf D., Ramirez S.I. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501.e1415. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le Bert N., Tan A.T., Kunasegaran K. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 45.Braun J., Loyal L., Frentsch M. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 46.Gallais F., Velay A., Wendling M.J. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis. 2021;27(1):113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Honke N., Shaabani N., Merches K. Immunoactivation induced by chronic viral infection inhibits viral replication and drives immunosuppression through sustained IFN-I responses. Eur J Immunol. 2016;46(2):372–380. doi: 10.1002/eji.201545765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ng K.W., Faulkner N., Cornish G.H. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science. 2020;370(6522):1339–1343. doi: 10.1126/science.abe1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagar M., Reifler K., Rossi M. Recent endemic coronavirus infection is associated with less severe COVID-19. J Clin Invest. 2021;131(1):e143380. doi: 10.1172/JCI143380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Villa Lario A., Vega-Díez D., González-Cañete M. Histological findings in chilblain-lupus like COVID lesions: in search of an answer to understand their aetiology. J Eur Acad Dermatol Venereol. 2020;34(10):e572–e574. doi: 10.1111/jdv.16733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang M.L., Chan M.P. Comparative analysis of chilblain lupus erythematosus and idiopathic perniosis: histopathologic features and immunohistochemistry for CD123 and CD30. Am J Dermatopathol. 2018;40(4):265–271. doi: 10.1097/DAD.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 52.Battesti G., El Khalifa J., Abdelhedi N. New insights in COVID-19-associated chilblains: a comparative study with chilblain lupus erythematosus. J Am Acad Dermatol. 2020;83(4):1219–1222. doi: 10.1016/j.jaad.2020.06.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aschoff R., Zimmermann N., Beissert S., Günther C. Type I interferon signature in chilblain-like lesions associated with the COVID-19 pandemic. Dermatopathology (Basel) 2020;7(3):57–63. doi: 10.3390/dermatopathology7030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hubiche T., Cardot-Leccia N., Le Duff F. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. https://doi.org/10.1001/jamadermatol.2020.4324 [published online ahead of print November 25, 2020]. JAMA Dermatol. [DOI] [PMC free article] [PubMed]
- 55.Torrelo A., Andina D., Santonja C. Erythema multiforme-like lesions in children and COVID-19. Pediatr Dermatol. 2020;37(3):442–446. doi: 10.1111/pde.14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colmenero I., Santonja C., Alonso-Riaño M. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultraestructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santonja C., Heras F., Núñez L., Requena L. COVID-19 chilblain-like lesion: immunohistochemical demonstration of SARS-CoV-2 spike protein in blood vessel endothelium and sweat gland epithelium in a polymerase chain reaction-negative patient. Br J Dermatol. 2020;183(4):778–780. doi: 10.1111/bjd.19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ko C.J., Harigopal M., Damsky W. Perniosis during the COVID-19 pandemic: negative anti-SARS-CoV-2 immunohistochemistry in six patients and comparison to perniosis before the emergence of SARS-CoV-2. J Cutan Pathol. 2020;47(11):997–1002. doi: 10.1111/cup.13830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ko C.J., Harigopal M., Gehlhausen J.R., Bosenberg M., McNiff J.M., Damsky W. Discordant anti-SARS-CoV-2 spike protein and RNA staining in cutaneous perniotic lesions suggests endothelial deposition of cleaved spike protein. J Cutan Pathol. 2020;48(1):47–52. doi: 10.1111/cup.13866. [DOI] [PubMed] [Google Scholar]
- 60.Gianotti R., Coggi A., Boggio F., Fellegara G. Similarities in cutaneous histopathological patterns between COVID-19-positive and COVID-19 high-risk patients with skin dermatosis. Acta Derm Venereol. 2020;100(15):adv00249. doi: 10.2340/00015555-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herman A., Peeters C., Verroken A. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156(9):998–1003. doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gambichler T., Reuther J., Stücker M. SARS-CoV-2 spike protein is present in both endothelial and eccrine cells of a chilblain-like skin lesion. https://doi.org/10.1111/jdv.16970 [published online ahead of print October 1, 2020]. J Eur Acad Dermatol Venereol. [DOI] [PMC free article] [PubMed]
- 63.Lipsker D. Paraviral eruptions in the era of COVID-19: do some skin manifestations point to a natural resistance to SARS-CoV-2? Clin Dermatol. 2020;38(6):757–761. doi: 10.1016/j.clindermatol.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W., Moore M.J., Vasilieva N. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glowacka I., Bertram S., Müller M.A. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J., Sriramula S., Xia H. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ Res. 2017;121(1):43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88(2):1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Y., Zhou R., Zhang H. Skin is a potential host of SARS-CoV-2: a clinical, single-cell transcriptome-profiling and histologic study. J Am Acad Dermatol. 2020;83(6):1755–1757. doi: 10.1016/j.jaad.2020.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peckham H., de Gruijter N.M., Raine C. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hilliard L.M., Sampson A.K., Brown R.D., Denton K.M. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep. 2013;15(1):71–79. doi: 10.1007/s11906-012-0319-y. [DOI] [PubMed] [Google Scholar]
- 77.Lang J.A., Krajek A.C. Age-related differences in the cutaneous vascular response to exogenous angiotensin II. Am J Physiol Heart Circ Physiol. 2019;316(3):H516–H521. doi: 10.1152/ajpheart.00509.2018. [DOI] [PubMed] [Google Scholar]
- 78.Mompeón A., Lázaro-Franco M., Bueno-Betí C. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1-7 production. Mol Cell Endocrinol. 2016;422:1–8. doi: 10.1016/j.mce.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 79.Vanhoutte P.M., Zhao Y., Xu A., Leung S.W.S. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res. 2016;119(2):375–396. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 80.Wambier C.G., Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83(1):308–309. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ren J., Nie Y., Lv M. Estrogen upregulates MICA/B expression in human non-small cell lung cancer through the regulation of ADAM17. Cell Mol Immunol. 2015;12(6):768–776. doi: 10.1038/cmi.2014.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucas J.M., Heinlein C., Kim T. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schuler B.A., Habermann A.C., Plosa E.J. Age-determined expression of priming protease TMPRSS2 and localization of SARS-CoV-2 in lung epithelium. J Clin Invest. 2021;131(1):e140766. doi: 10.1172/JCI140766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou M., Dai W., Cui Y., Li Y. Estrogen downregulates gp130 expression in HUVECs by regulating ADAM10 and ADAM17 via the estrogen receptor. Biochem Biophys Res Commun. 2020;523(3):753–758. doi: 10.1016/j.bbrc.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Schumacher N., Rose-John S. ADAM17 activity and IL-6 trans-signaling in inflammation and cancer. Cancers (Basel) 2019;11(11):1736. doi: 10.3390/cancers11111736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ziegler S.M., Beisel C., Sutter K. Human pDCs display sex-specific differences in type I interferon subtypes and interferon α/β receptor expression. Eur J Immunol. 2017;47(2):251–256. doi: 10.1002/eji.201646725. [DOI] [PubMed] [Google Scholar]
- 87.Webb K., Peckham H., Radziszewska A. Sex and pubertal differences in the type 1 interferon pathway associate with both X chromosome number and serum sex hormone concentration. Front Immunol. 2018;9:3167. doi: 10.3389/fimmu.2018.03167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Souyris M., Cenac C., Azar P. TLR7 escapes X chromosome inactivation in immune cells. Sci Immunol. 2018;3(19):eaap8855. doi: 10.1126/sciimmunol.aap8855. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi T., Ellingson M.K., Wong P. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van der Made C.I., Simons A., Schuurs-Hoeijmakers J. Presence of genetic variants among young men with severe COVID-19. JAMA. 2020;324(7):1–11. doi: 10.1001/jama.2020.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Q., Bastard P., Liu Z. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pairo-Castineira E., Clohisey S., Klaric L. Genetic mechanisms of critical illness in Covid-19. https://doi.org/10.1038/s41586-020-03065-y [published online ahead of print December 11, 2020]. Nature.
- 93.Bastard P., Rosen L.B., Zhang Q. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agrawal A. Mechanisms and implications of age-associated impaired innate interferon secretion by dendritic cells: a mini-review. Gerontology. 2013;59(5):421–426. doi: 10.1159/000350536. [DOI] [PubMed] [Google Scholar]
- 95.Kim O.Y., Chae J.S., Paik J.K. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age (Dordr) 2012;34(2):415–425. doi: 10.1007/s11357-011-9244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maggio M., Basaria S., Ble A. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 97.Gao Y., Li T., Han M. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pierce C.A., Preston-Hurlburt P., Dai Y. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci Transl Med. 2020;12(564):eabd5487. doi: 10.1126/scitranslmed.abd5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weisberg S.P., Connors T.J., Zhu Y. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2020;22(1):25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. https://doi.org/10.1136/archdischild-2020-320338 [published online ahead of print December 1, 2020]. Arch Dis Child. [DOI] [PubMed]
- 101.Jamilloux Y., Henry T., Belot A. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19(7):102567. doi: 10.1016/j.autrev.2020.102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reizis B. Plasmacytoid dendritic cells: development, regulation, and function. Immunity. 2019;50(1):37–50. doi: 10.1016/j.immuni.2018.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Assil S., Coléon S., Dong C. Plasmacytoid dendritic cells and infected cells form an interferogenic synapse required for antiviral responses. Cell Host Microbe. 2019;25(5):730–745.e736. doi: 10.1016/j.chom.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 104.Barrat F.J., Su L. A pathogenic role of plasmacytoid dendritic cells in autoimmunity and chronic viral infection. J Exp Med. 2019;216(9):1974–1985. doi: 10.1084/jem.20181359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cervantes-Barragan L., Lewis K.L., Firner S. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc Natl Acad Sci U S A. 2012;109(8):3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang F., Nie J., Wang H. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuen C.K., Lam J.Y., Wong W.M. SARS-CoV-2 nsp13, nsp14, nsp15 and orf6 function as potent interferon antagonists. Emerg Microbes Infect. 2020;9(1):1418–1428. doi: 10.1080/22221751.2020.1780953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hadjadj J., Yatim N., Barnabei L. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ziegler C.G.K., Allon S.J., Nyquist S.K., HCA Lung Biological Network SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181(5):1016–1035.e1019. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bowles L., Platton S., Yartey N. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med. 2020;383(3):288–290. doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18(8):2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y., Cao W., Jiang W. Profile of natural anticoagulant, coagulant factor and anti-phospholipid antibody in critically ill COVID-19 patients. J Thromb Thrombolysis. 2020;50(3):580–586. doi: 10.1007/s11239-020-02182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zuo Y., Estes S.K., Ali R.A. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med. 2020;12(570):eabd3876. doi: 10.1126/scitranslmed.abd3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mendoza-Pinto C., García-Carrasco M., Cervera R. Role of infectious diseases in the antiphospholipid syndrome (including its catastrophic variant) Curr Rheumatol Rep. 2018;20(10):62. doi: 10.1007/s11926-018-0773-x. [DOI] [PubMed] [Google Scholar]
- 116.Lutz V., Cribier B., Lipsker D. Chilblains and antiphospholipid antibodies: report of four cases and review of the literature. Br J Dermatol. 2010;163(3):645–646. doi: 10.1111/j.1365-2133.2010.09829.x. [DOI] [PubMed] [Google Scholar]
- 117.Droesch C., Do M.H., DeSancho M., Lee E.J., Magro C., Harp J. Livedoid and purpuric skin eruptions associated with coagulopathy in severe COVID-19. JAMA Dermatol. 2020;156(9):1–3. doi: 10.1001/jamadermatol.2020.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marchandot B., Sattler L., Jesel L. COVID-19 related coagulopathy: a distinct entity? J Clin Med. 2020;9(6):1651. doi: 10.3390/jcm9061651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Helms J., Tacquard C., Severac F. CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Senchenkova E.Y., Russell J., Esmon C.T., Granger D.N. Roles of coagulation and fibrinolysis in angiotensin II-enhanced microvascular thrombosis. Microcirculation. 2014;21(5):401–407. doi: 10.1111/micc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Senchenkova E.Y., Russell J., Kurmaeva E., Ostanin D., Granger D.N. Role of T lymphocytes in angiotensin II-mediated microvascular thrombosis. Hypertension. 2011;58(5):959–965. doi: 10.1161/HYPERTENSIONAHA.111.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang Y., Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179(2):137–145. doi: 10.1111/cei.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Senchenkova E.Y., Russell J., Yildirim A., Granger D.N., Gavins F.N.E. Novel role of T cells and IL-6 (interleukin-6) in angiotensin II-induced microvascular dysfunction. Hypertension. 2019;73(4):829–838. doi: 10.1161/HYPERTENSIONAHA.118.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Morris R., Kershaw N.J., Babon J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984–2009. doi: 10.1002/pro.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Z.W., Tsai C.H., Pan C.T., TAIPAI Study Group Endothelial dysfunction in primary aldosteronism. Int J Mol Sci. 2019;20(20):5214. doi: 10.3390/ijms20205214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herrada A.A., Contreras F.J., Marini N.P. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J Immunol. 2010;184(1):191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 128.Meng Y., Chen C., Liu Y., Tian C., Li H.H. Angiotensin II regulates dendritic cells through activation of NF-kappaB /p65, ERK1/2 and STAT1 pathways. Cell Physiol Biochem. 2017;42(4):1550–1558. doi: 10.1159/000479272. [DOI] [PubMed] [Google Scholar]
- 129.Cavezzi A., Troiani E., Corrao S. COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2):1271. doi: 10.4081/cp.2020.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dang E.V., Barbi J., Yang H.Y. Control of TH17/Treg balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jahani M., Dokaneheifard S., Mansouri K. Hypoxia: a key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J Inflamm (Lond) 2020;17(1):33. doi: 10.1186/s12950-020-00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Steckelings U.M., Wollschläger T., Peters J., Henz B.M., Hermes B., Artuc M. Human skin: source of and target organ for angiotensin II. Exp. Dermatol. 2004;13(3):148–154. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 133.Lang J.A., Kolb K.E. Angiotensin II type I receptor blockade attenuates reflex cutaneous vasoconstriction in aged but not young skin. Am J Physiol Heart Circ Physiol. 2015;308(10):H1215–H1220. doi: 10.1152/ajpheart.00017.2015. [DOI] [PubMed] [Google Scholar]
- 134.Stephens D.P., Aoki K., Kosiba W.A., Johnson J.M. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol. 2001;280(4):H1496–H1504. doi: 10.1152/ajpheart.2001.280.4.H1496. [DOI] [PubMed] [Google Scholar]
- 135.Greaney J.L., Stanhewicz A.E., Kenney W.L., Alexander L.M. Impaired increases in skin sympathetic nerve activity contribute to age-related decrements in reflex cutaneous vasoconstriction. J Physiol. 2015;593(9):2199–2211. doi: 10.1113/JP270062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Forrester S.J., Booz G.W., Sigmund C.D. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98(3):1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Verheyden M., Grosber M., Gutermuth J., Velkeniers B. Relapsing symmetric livedo reticularis in a patient with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020;34(11):e684–e686. doi: 10.1111/jdv.16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pulia M.S., O’Brien T.P., Hou P.C., Schuman A., Sambursky R. Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic. Ann Med. 2020;52(5):207–214. doi: 10.1080/07853890.2020.1763449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Melgaço J.G., Azamor T., Ano Bom A.P.D. Protective immunity after COVID-19 has been questioned: what can we do without SARS-CoV-2-IgG detection? Cell Immunol. 2020;353:104114. doi: 10.1016/j.cellimm.2020.104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.