To the Editor:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has spread rapidly around the world since first identified in Wuhan, China, in December 2019.1 Long-term care residents have been among the hardest impacted by COVID-19. More than 500,000 residents in nursing homes and long-term care facilities have been infected in the United States, accounting for 40% of the total deaths from COVID-19.2 Among survivors, an understanding of the expected durability of the antibody response may be helpful in estimating the risk of a new infection. This is particularly important in preparation for another surge. Previous studies have reported durability of response up to 4 months in adults with an average age ≤60 years.3, 4, 5 However, little is known about the durability of antibody response in older, potentially less immunogenic long-term care populations.6 , 7 We assessed the durability of antibody response in our long-term care population from the time of initial infection over a 6-month period.
Methods
The VA Boston Healthcare System includes a 112-bed skilled nursing facility for Veterans needing long-term care. All residents had nasal SARS-CoV-2 polymerase chain reaction (PCR) testing performed at baseline when all were asymptomatic and negative, and then again for symptoms and for surveillance at repeated intervals. Serum antibody testing was also performed after the first surge and then again periodically over the following 6 months as part of routine surveillance.
The SARS-CoV-2 IgG assay was performed on the Abbott Architect i2000SR (Abbott Diagnostics, Chicago, IL). This assay is a chemiluminescent immunoassay that detects IgG against the nucleocapsid protein of SARS-CoV-2. It received EUA by the FDA in March 2020 and was available in our laboratory in April 2020. It has been demonstrated to have 99.9% specificity and 100% sensitivity for detecting the IgG antibody in patients 17 days or more after symptom onset.8 As per manufacturer instructions, a signal/cut-off (S/CO) ratio of greater than or equal to 1.4 was interpreted as reactive, and an S/CO ratio of less than 1.4 was interpreted as nonreactive. The change in this index value over time was measured from the time of PCR diagnosis for each individual. This work was exempt from local institutional review board approval.
Results
In a population of 30 residents in a single long-term care unit who were all negative by PCR at baseline, 24 were infected with COVID-19 during the month of April 2020. All residents had at least 1 clinical symptom at the time of diagnosis by PCR. Hospitalization was required in 11 (46%), and 8 (33%) died.
Of these 24 COVID-19–positive patients, 15 had antibody testing performed sometime during the 6-month period of the study; 9 were unavailable for testing. The 6 residents not diagnosed with COVID-19 did not have clinical symptoms of COVID-19 infection and had negative PCR tests; all also had negative antibody testing.
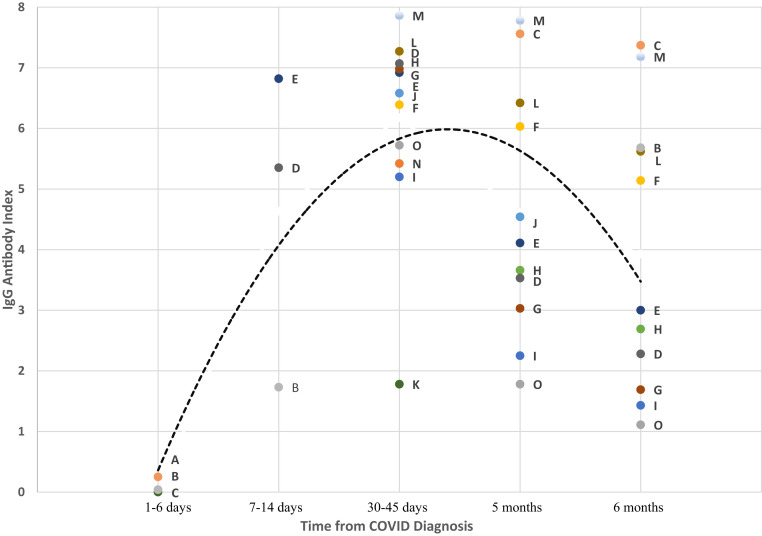
Among the 15 COVID-19–positive residents with antibody testing performed, 3 had tests in 1–6 days from the time of infection diagnosis and all 3 had negative results. By days 7–14, all residents who were tested had antibodies detected. By 6 months, 10 of 11 residents tested still had detectable antibodies, although the index value was lower for all. There were 2 veterans who were immunosuppressed who continued to have detectable antibodies at 6 months. The 1 patient who had waning antibody response at 5 months and a negative index value at 6 months had mild symptoms throughout his course, including absence of fever. The pattern of antibody values is shown in Figure 1 . Every resident followed a similar pattern, with peak response at 30–45 days and declining response after that.
Fig. 1.
Durability of SARS-CoV-2 IgG antibody response in 15 COVID-19–positive long-term care patients over 6 months. Black dashed line represents the average SARS-CoV-2 IgG antibody response. Each patient is represented by a unique colored point and letter combination across the figure. The IgG antibody index to SARS-CoV-2 is determined by comparing the chemiluminescent relative light unit (RLU) in the reaction to the calibrator RLU. The assay is an automated, 2-step immunoassay for the qualitative detection of IgG antibodies to SARS-CoV-2 in human serum and plasma using chemiluminescent microparticle immunoassay technology.
Discussion
The SARS-CoV-2 IgG assay is helpful in identifying recent or prior COVID-19 infection. At this time, it is unknown how long antibodies persist following infection. In this population of long-term care residents, we found that 91% still had IgG seropositivity at 6 months, although with lower titers over time. Others have shown that individuals with mild or asymptomatic infection develop less robust antibody responses, possibly accounting for the 1 patient who lost seropositivity at 6 months.9 The durability of response to natural infection in this vulnerable population clearly wanes over time, further magnifying the importance of the large vaccination efforts currently under way.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of COVID-19—studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 2.Kaiser Family Foundation https://www.kff.org/coronavirus-covid-19/issue-brief/state-data-and-policy-actions-to-address-coronavirus/ Available at:
- 3.Isho B., Abe K.T., Zuo M. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020;5:eabe5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer A.S., Jones F.K., Nodoushani A. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020;5:eabe0367. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel M.M., Thornburg N.J., Stubblefield W.B. Change in antibodies to SARS-CoV-2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324:1781–1782. doi: 10.1001/jama.2020.18796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladhani S.N., Jeffery-Smith A., Patel M. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: Prospective cohort study, England. SSRN Electron J. 2020;28:100597. doi: 10.1016/j.eclinm.2020.100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham NSN, Junghans C., McLaren R. High rates of SARS-CoV-2 seropositivity in nursing home residents. J Infect. 2020 doi: 10.1016/j.jinf.2020.08.040. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryan A., Pepper G., Wener M.H. Performance characteristics of the Abbott architect Sars-Cov-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58 doi: 10.1128/JCM.00941-20. e00941–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q.X., Tang X.J., Shi Q.L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]