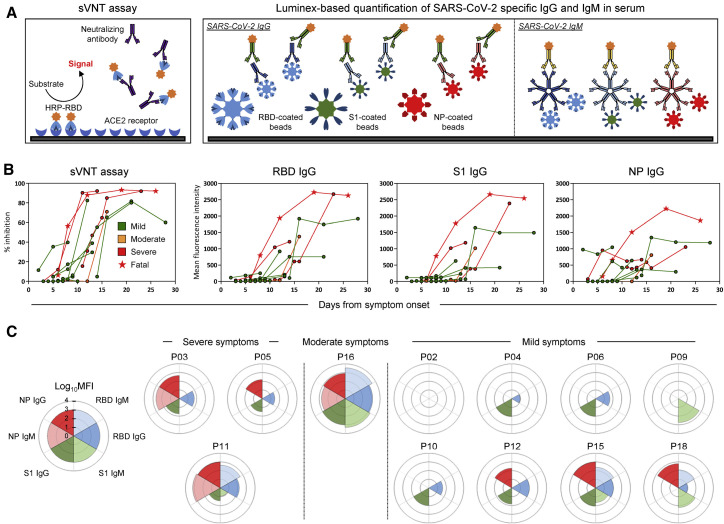
Figure 2.
Longitudinal analysis of SARS-CoV-2-specific antibody-related responses in acute COVID-19 patients
(A) Schematic representation of the surrogate virus neutralization assay and the Luminex-based assay to quantify SARS-CoV-2 RBD-, S1-, and NP-specific IgG and IgM antibodies. Cutoffs to define significant virus neutralization and antibody quantities were set at 20% inhibition for the sVNT assay (as defined in ref (Tan et al., 2020)) and MFI (mean fluorescence intensity) > 100 for the Luminex-based assay, respectively.
(B) SARS-CoV-2 neutralization and relative quantities of specific IgG and antibodies (n = 12).
(C) Rose plots represent the quantity of RBD-, S1-, and NP-specific IgG and IgM antibodies at first detectable antibody response (n = 12). Patient P02 had no detectable antibody response at 3, 4, 7, and 9 days after symptom onset for which samples were available.