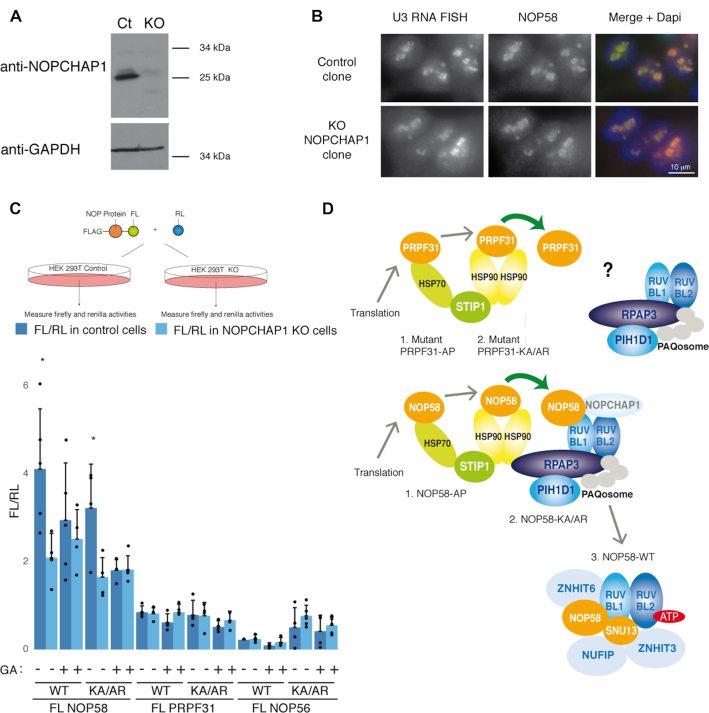
Figure 8.
NOPCHAP1 enhances expression of NOP58. (A) Characterization of a NOPCHAP1 KO clone generated in human HEK 293T cells. Western Blot with anti-NOPCHAP1 antibody using Laemmli extracts from NOPCHAP1 KO clone (KO) or control clone (Ct). Anti-GAPDH antibody was used as loading control. (B) The nucleolar structure is not affected in the NOPCHAP1 KO clone. Epifluorescence microscopy images of HEK 293T control and NOPCHAP1 KO clones. Red/left panels: RNA FISH with U3 specific probes. Green/ middle panels: IF with an anti-NOP58 antibody. Right panels: merge with DAPI staining. Scale bar is 10 μm. (C) Top panel: schematic representation of the assay. Bottom panel: graph showing the ratio FL/RL representing the expression of indicated FL-fusion protein in control cells (dark blue) and NOPCHAP1 KO cells (light blue), in normal conditions or after Geldanamycin treatment (GA) for 12 hr. The WT or mutant forms of protein used in the assay is indicated below the graph. A co-transfected plasmid expressing RL alone was used to normalize the value obtained for FL-fusion proteins. The values are means of four experiments that are each represented by a dot. Error bars: standard deviation. * P-value ≤ 0.05 according to a Student test. (D) Model for the assembly of PRPF31 and NOP58 and specific role of NOPCHAP1 in box C/D snoRNP biogenesis. Newly-synthesized NOP58 associates with HSP70 and HSP90, and is transferred to R2TP by NOPCHAP1. A SNU13/ZNHIT3/NUFIP1 module binds R2TP via PIH1D1, and this leads to the assembly of NOP58 with SNU13, maintained by ATP-loaded RUVBL1/2, ZNHIT6 and NUFIP1/ZNHIT3. In the case of PRPF31, it is unclear how this protein associates with R2TP. The steps where NOP58 and PRPF31 mutants accumulate are indicated.